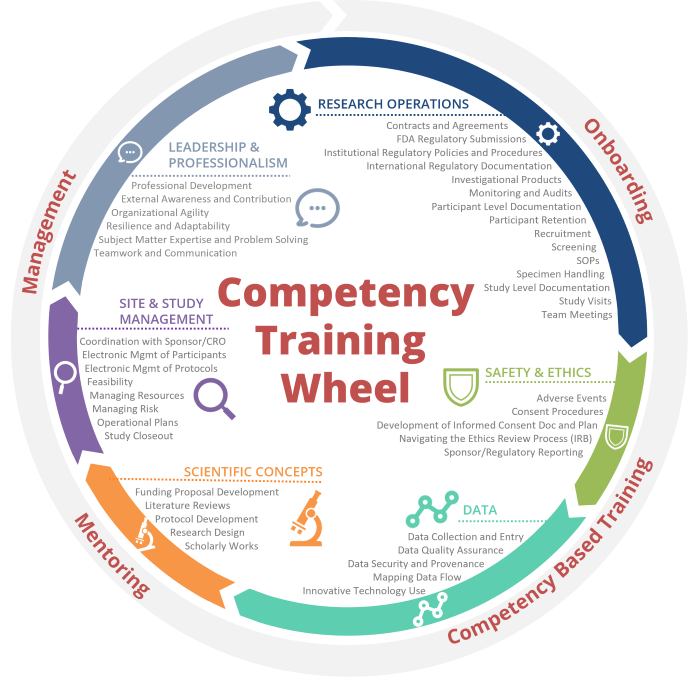
Training by Competency:
This is a list of course offerings mapped to the Duke WE-R clinical research competencies. A full list of the competencies and associated assessments for tier advancement can be found on the WE-R Tier Advancement page.
If you encounter any broken links we missed in an online module, please let us know using this form.
Jump to: Research Operations | Safety and Ethics | Data | Scientific Concepts | Site and Study Management | Leadership and Professionalism | RPL Competencies
Duke has purchased the all-access webinar package from CITI. There are over 120 webinars to choose from on various research topics! Here is a tip sheet for adding the webinars to your Duke Health CITI Account.
You can find webinars that are currently available listed on the CITI Website. Additionally, DOCR has organized them all by clinical research competency and domain for you in this spreadsheet. The spreadsheet can be filtered by domain, competency, or webinar title.

- Leading the day-to-day research operations of clinical research studies conducted by the principal investigators.
- Being knowledgeable in regulatory and institutional policies and processes and applying this knowledge to study documentation, protocol submissions, and standard operating procedures (SOPs).
- Screening and consenting participants for research studies. Maintaining participant level and study level documentation.
- Preparing for and providing support for study monitoring and audit visits, including support for the reviewer.
Engagement Activity Packet: Contracts and Agreements
(full list of packets is available on Onboarding Learning Plan page)
Research Related Agreements: The Basics for Clinical Research Study Teams | 00152721
This online basics course is an overview of typical clinical research-related contracts and agreements and how they are handled at Duke, along with an introduction to the Duke Office of Research Contracts (ORC).
Course Objectives:
- Identify typical agreements/contracts (MTAs, CTAs, CDAs, DTAs)
- Recognize when typical agreements are necessary
- Describe processes for putting agreements in place
- Discuss tips and tricks for designing databases for research purposes
- Identify special considerations and tips when establishing an agreement
Industry Funded Clinical Research - Process for Contracts | RCC Module for Financial Staff
This course presents information regarding the contract approval process for industry-supported research, and details best practices for preparing a successful SPS entry for industry-sponsored research projects.
Course Objectives:
- Outline information regarding the contract approval process for industry-supported research that will enable getting the contract signed
- Detail best practices for preparing a successful SPS entry for industry-supported research projects
Investigational New Drug Sponsor and Investigator Responsibilities | 00122655
This training will cover the responsibilities associated with maintaining an IND and is intended for academic investigators who will hold an IND. This training consists of ten modules. The first nine modules will cover IND sponsor responsibilities that must be fulfilled by investigators who conduct a clinical investigation run under an IND. Investigators fulfilling a dual role as sponsor and investigator, in other words, sponsor-investigators, must fulfill the responsibilities of both sponsors and investigators.
The Modules include:
- Introduction to IND Sponsors and Investigator Responsibilities
- Overview of IND Maintenance and Tracking
- IND Annual Reports
- IND Safety Reporting
- IND Information and Amendments
- Maintaining Accountability Records for INDs
- IND Protocol Amendments
- IND Monitoring and Multi-Center Trails
- Financial Disclosure
- IND Investigator Responsibilities
Investigational Device Exemption Sponsor and Investigator Responsibilities | 00122655
This training will cover the responsibilities associated with maintaining an IDE and is intended for academic investigators who will hold that IDE for a significant risk device study. This training consists of ten modules. The first nine modules will cover IDE sponsor responsibilities and the final module will cover responsibilities that must be fulfilled by investigators who conduct a significant risk device study run under an IDE. Investigators fulfilling a dual role as sponsor and investigator, in other words, sponsor-investigators, must fulfill the responsibilities of both sponsors and investigators.
The Modules include:
- Introduction to IDE Sponsor and Investigator Responsibilities
- Overview of IDE Maintenance and Tracking
- IDE Progress Reports and Final Reports
- IDE Modifications
- Unanticipated Adverse Device Effects
- IDE Reports
- Maintaining Accountability Records for IDEs
- IDE Monitoring and Multi-Center Trails
- Financial Disclosure
- IDE Investigator Responsibilities
Regulating Medical Products: FDA Oversight of Drugs and Devices | 00151992
This course provides an overview of how the FDA regulates drugs and medical devices that are tested using clinical trials.
Office of Regulatory Affairs and Quality
Regulatory Affairs Training Program
CITI Training
- ICH - Comparison Between ICH GCP E6 and U.S. FDA Regulations
- The CITI Good Clinical Practice Course for Clinical Trials with Investigational Drugs and Medical Devices (U.S. FDA Focus)
- Investigator Obligations in FDA-Regulated Research
- Overview of U.S. FDA Regulations for Medical Devices
These modules are included in the required CITI Good Clinical Practice course. If you have already taken CITI and want to find these modules: log into your Duke Health CITI profile at https://about.citiprogram.org/ and click “View Courses” next to Duke Health. Then click “Review Course” next to the CITI Good Clinical Practice course. Read more about CITI training requirements here.
Engagement Activity Packet: Institutional Regulatory Policies and Processes
(full list of packets is available on Onboarding Learning Plan page)
Institutional Regulatory Policies and Procedures in Clinical Research Management - DOCR | 00139987
This course will provide Duke employees engaged in clinical research with information about regulations, guidelines, and policies for conducting clinical research at Duke University.
Learning Objectives:
- Find and follow applicable standards, guidelines, regulations, policies and procedures for performing clinical research at Duke
- Identify and interpret policies and regulations regarding Duke Health IRB oversight of research
- Describe study elements that must be approved by or reported to the Duke Health IRB
- Identify research approval elements and responsible offices at Duke
IRB Overview - DOCR-RES-300
This course offers an overview of the DUHS IRB review process and requirements, federal regulations, and Duke policies pertaining to research involving human subjects. The course highlights tools available to study teams on the IRB web site; presents an example of an eIRB submission; and provides strategies for working efficiently with the IRB.
Course Objectives:
- Describe the DUHS IRB’s scope of oversight – what does and what does not require review by the DUHS IRB
- Describe the IRB review process
- Outline a basic overview of the eIRB system, including how to gain access to eIRB, how to use and navigate within eIRB, how to find out study status, and how to get help with eIRB
- Describe reporting to the IRB
- Describe how to communicate with the IRB and make use of the many tools available on the IRB website
Navigating Clinical Research at Duke: What You Need to Know - DOCR | 00141224
This course is part of the Express Start onboarding collection. It will provide the general organizational structure of Duke, central support offices, and an overview of a Clinical Research Unit (CRU) and Oversight Organization (OO).
Prompt Reporting to the IRB WBT - DOCR | 00148966
This 10 minute online module will walk you through protocol deviations and their counterparts protocol violations, adverse events and unanticipated problems, and the subsequent steps of reporting these events to the IRB.
Course Objectives:
- Recognize what constitutes a protocol deviation, violation, adverse event, and unanticipated problem
- Recognize what events need to be reported to the IRB
- Identify FDAAA required informed consent language
- IRecall the reporting timeframe for these events
- Attest to abiding by the policy requirements detailed in Problems or Events that Require Prompt Reporting to the IRB.
Study Documentation Regulations and Best Practices - DOCR | 00137344
This course outlines the required components of study documentation for all clinical research, defines standard documentation terminology, and applies knowledge of documentation best practices to everyday scenarios faced by study teams.
Course Objectives:
- Outline required components of study documentation for all clinical research
- Distinguish between documentation regulations and best practice
- Define standard documentation terminology
- Apply knowledge of documentation best practices to day-to-day documentation scenarios faced by study teams
Engagement Activity Packet: Investigational Products
(full list of packets is available on Onboarding Learning Plan page)
Investigational Product Management and Accountability for the Study Team | 00178713 | Online
This online course is intended to help clinical research personnel get started working with investigational products at Duke. Clinical research staff may be responsible for different aspects of managing and documenting an Investigational Product (IP) or Investigational Study Drug (ISD); including arrival, storage, tracking, provision to research participants, and (if applicable) return and/or destruction. These roles may also serve as the primary coordinator with study sponsors, Duke Department of Pharmacy Investigational Drug Services (IDSs), and other parties as necessary.
Learning Objectives:
- Identify and interpret policies for administration of investigational products (IP)
- Recognize when an Investigational New Drug (IND) or Investigational Device Exemption (IDE) application is needed
- Describe study design requirements for studies involving IP
- Describe potential documentation requirements related to IP
- Identify personnel responsible for various IP management activities
- Describe requirements and processes for using the Duke Investigational Drug Services (IDSs)
Regulating Medical Products: FDA Oversight of Drugs and Devices | 00151992
This course provides an overview of how the FDA regulates drugs and medical devices that are tested using clinical trials.
CITI Training
- Overview of New Drug Development
- Conducting Investigator-Initiated Studies According to FDA Regulations and GCP
- The CITI Good Clinical Practice Course for Clinical Trials with Investigational Drugs and Medical Devices (U.S. FDA Focus)
- Managing Investigational Agents According to GCP Requirements
- Overview of U.S. FDA Regulations for Medical Devices
These modules are included in the required CITI Good Clinical Practice course. If you have already taken CITI and want to find these modules: log into your Duke Health CITI profile at https://about.citiprogram.org/ and click “View Courses” next to Duke Health. Then click “Review Course” next to the CITI Good Clinical Practice course. Read more about CITI training requirements here.
Investigational Products (Provided by the Society of Clinical Research Sites)
Overview of the basics of Investigational Product management and use during a clinical trial. Topics include inventory and temperature control and recommendations for source documentation.
DOCR Research Wednesday recording on OARC Updates (5/10/23)
CITI Training
- Monitoring of Clinical Trials by Industry Sponsors
- Audits and Inspections of Clinical Trials
These modules are included in the required CITI Good Clinical Practice course. If you have already taken CITI and want to find these modules: log into your Duke Health CITI profile at https://about.citiprogram.org/ and click “View Courses” next to Duke Health. Then click “Review Course” next to the CITI Good Clinical Practice course. Read more about CITI training requirements here.
Monitoring and Auditing (Provided by the Society of Clinical Research Sites)
Describes the routine monitoring and auditing activities that occur during a clinical study. This topic includes information on the purpose, what to expect, and tips to prepare for these Sponsor activities.
Study Documentation Refresher Series 3: Participant Level Documentation | 00145567
This 15 to 20 minute module discusses what documents are “participant level” along with some specifics around informed consent, research data, and source data documentation. You’ll also learn how case report forms are used to report research data to the sponsor.
Study Documentation Regulations and Best Practices - DOCR | 00137344
This course outlines the required components of study documentation for all clinical research, defines standard documentation terminology, and applies knowledge of documentation best practices to everyday scenarios faced by study teams.
Course Objectives:
- Outline required components of study documentation for all clinical research
- Distinguish between documentation regulations and best practice
- Define standard documentation terminology
- Apply knowledge of documentation best practices to day-to-day documentation scenarios faced by study teams
Interventional Study Eligibility Documentation | 00190949
This training is for investigators and research teams at Duke who will create eligibility source documentation for interventional clinical research studies that are open to participant accrual. Interventional clinical research includes studies with an OnCore protocol type of Treatment, Prevention, Device Feasibility, Diagnostic, Screening or Supportive Care.
Learning Objectives
-
Define study eligibility determination.
-
Document study eligibility using two appropriate methods.
-
Attest to your understanding of Duke policy related to documenting study eligibility.
OnCore Training for the Clinical Research Coordinator - DOCR | 00110056
This online training course will provide step by step instructions on completing specific tasks relevant to the basic function(s) of a Clinical Research Coordinator at Duke.
This module covers:
- Subject Administration
- Subject Calendars & Tracking
- Reporting
OnCore Training for the Regulatory Coordinator - DOCR | 00114396
This online training course will provide step by step instructions on completing specific tasks relevant to the basic function(s) of a Clinical Research Regulatory Coordinator at Duke.
This module covers:
- Regulatory Workflows at Duke
- Using the PC Console
- Reporting Functions
MC Clinical Research 100 E-Learning Module | 00129623
This module provides instruction for new clinical researchers who will work in a Clinical Research Coordinator (CRC) user role or as a refresher for existing clinical research staff. Research Dashboard and system functionality will be reviewed in the module. This course, as well as passing an end-of-course test, is a requirement for CRC access to Maestro Care.
This module covers:
- The Research Dashboard
- Enrolling Patients in Studies
- Order Management
- Encounters-linking
- Conducting and Documenting a Patients Study Visit
- Navigating the In Patient Record- Chart Review
Essential Documents for a Clinical Study (Provided by the Society of Clinical Research Sites)
Describes the requirements for Clinical Study essential documents. Describes the type of essential documents collected for a Clinical Study during the different study phases.
Source Documentation (Provided by the Society of Clinical Research Sites)
A review of the terms source data and source documents. Describes the key attributes of source documents, the intent of ALCOA and CCEA and describes the appropriate processes for creating, maintaining and storing source documents.
Strategies to Support Retention of Clinical Research Participants | DOCR | 00161197
This new online module is now available in the LMS! This module was developed in collaboration with the RIC, DOCR, and CERI and takes about 20 minutes to complete. The purpose of this online module is to reviews the strategies clinical research teams can implement to support the retention of clinical research participants. There are resources and tips shared within this module that will help clinical research personnel work through this critical part of their role.
Learning Objectives:
- Define participant retention
- Describe strategies for retention and their importance
- Identify ways to set clear study expectations to support retention
- Discuss the importance of study continuation with participants
Readability Fundamentals for Clinical Research Participant Engagement Materials; DOCR | 00160856
The purpose of this online module is to provide clinical research staff with the foundational information needed to create readable engagement materials for clinical research studies. There are resources, tools, and tips within this module that will help clinical research personnel work through this critical part of their role.
Learning Objectives:
- Define readability and health literacy
- Recognize the fundamentals of readability in the context of clinical research
- Recognize the importance of producing materials that participants can understand
- Identify ways to assess the readability of materials and confirm participant understanding
Recruitment Innovation Center through CTSI | On-demand
Plain Language Mini-Consults: Our plain language mini-consult will provide you with a lay-summary of your research, bulleted statements and taglines that you can use in your advertising materials and a lay-friendly concise summary of your project that you can use in your informed consent form.
Engagement Activity Packet: Recruitment and Screening
(full list of packets is available on Onboarding Learning Plan page)
Clinical Research Recruitment Regulations and Tools - DOCR | 00153576
The course is designed to provide study teams with an overview of recruitment planning at Duke. The course includes information about the Engagement Policy, obtaining IRB approval for your recruitment plans and materials, branding requirements when you’re targeting Duke Health patients, and Maestro Care tools that support your efforts to identify people who are eligible for your studies.
- Discuss Duke policies related to recruitment and engagement.
- Recognize the importance of planning for recruitment.
- Recognize the process for obtaining IRB approval for recruitment plans and materials.
- Recall branding requirements when recruiting Duke Health patients.
- Discuss Maestro Care tools as a means to identify eligible participants.
Readability Fundamentals for Clinical Research Participant Engagement Materials; DOCR | 00160856
The purpose of this online module is to provide clinical research staff with the foundational information needed to create readable engagement materials for clinical research studies. There are resources, tools, and tips within this module that will help clinical research personnel work through this critical part of their role.
Learning Objectives:
- Define readability and health literacy
- Recognize the fundamentals of readability in the context of clinical research
- Recognize the importance of producing materials that participants can understand
- Identify ways to assess the readability of materials and confirm participant understanding
Recruitment and Engagement Policy Training -DOCR | 00135223
The Duke University Health System (DUHS) has revised language in the Notice of Privacy Practices (NPP) Brochure. To align with the revised NPP, the Recruitment and Engagement Policy will allow study teams to engage patients through contacts including, but not limited to portal messages, mobile health devices, telephone, electronic surveys at clinic visits or hospital admissions, or directly via one or more clinicians. Any of these methods may be used to engage patients in research related to their health, if approved by the DUHS Institutional Review Board (IRB). Training on the new policy is required prior to IRB approving updated recruitment plans utilizing expanded recruitment options.
Using Social Media for Study Recruitment: Questions to Consider | 00161752
This module will describe the purpose of recruitment using social media and provide an overview of questions to ask to help determine whether study teams should use social media as a recruitment tool.
Learning Objectives:
- Describe how social media is used for recruitment in research studies.
- Identify questions to determine whether social media is right for a study.
- Find policies and procedures related to using social media for recruitment at Duke.
Conducting a Study (Provided by the Society of Clinical Research Sites)
Explains the three stages of study conduct phases including the subject enrollment/recruitment period, the ongoing maintenance and the subject exit/completion phase including the tasks and activities associated with these stages.
Conducting a Study - Provided by the Society of Clinical Research Sites
Explains the three stages of study conduct phases including the subject enrollment/recruitment period, the ongoing maintenance and the subject exit/completion phase including the tasks and activities associated with these stages.
TBD
Phlebotomy Competency for Research | DOCR-RES-320 | In-person
This course provides training and verifies competency for research personnel (not otherwise licensed or certified) to perform venipuncture on adults. Upon completion of the course, personnel may perform venipuncture in a research environment at Duke Medicine. This course does not train the participant to be a phlebotomy technician or provide formal certification.
This course describes the process for safely completing a venipuncture, lists the supplies needed, the acceptable sites for venipuncture, the labeling process of tubes for Duke and other labs, the potential complications, and provides an opportunity to practice venipuncture under instructor supervision.
Phlebotomy Competency Training for Research Study Personnel - This policy defines the training requirements and skills necessary to perform venipuncture/phlebotomy for the purposes of research in Duke Medicine.
Manager approval required to attend this class.
Course Objectives:
- Describe the process for safely completing a venipuncture on an adult
- List the supplies needed to accomplish venipuncture
- Identify the common sites for venipuncture and sites not acceptable for venipuncture
- List the correct order of tubes for blood collection
- Describe the labeling process of tubes for both Duke and other labs
- Discuss potential complications
- Perform venipuncture with instructor supervision
Phlebotomy Renewal Competency for Research | DOCR-RES-350 | In-person
This competency renewal course verifies competency for research personnel (not otherwise licensed or certified) to perform venipuncture on adults. Upon completion of the course, personnel may perform venipuncture in a research environment at Duke Medicine. This course does not train the participant to be a phlebotomy technician or provide formal certification.
Phlebotomy Competency Training for Research Study Personnel (02/16/2024) - This policy defines the training requirements and skills necessary to perform venipuncture/phlebotomy for the purposes of research in Duke Medicine.
This course provides the renewal requirement for the Phlebotomy Competency for Research course. In order to renew this required learning, participants must:
- Attend a Phlebotomy Competency Renewal course
Course Objectives:
- Demonstrate phlebotomy competency on a test arm with an instructor present
- Demonstrate knowledge of phlebotomy through successful completion of an online quiz with 80% or greater accuracy
Urine Pregnancy Screening for Research | DOCR-RES-370 | In-person
This course outlines the requirements for conducting urine pregnancy screening for research and discusses how this differs from urine pregnancy testing performed for clinical care. Participants will practice pregnancy screening on the QuickVue OneStep. The QuickVue OneStep and the QuickVue OneStep + are the only DUHS IRB approved kits for pregnancy screening for research.
Urine Testing for Pregnancy Screening to Determine Eligibility to Participate in Research Studies
Course Objectives:
- Outline the requirements for conducting urine pregnancy screening for research
- Discuss how urine pregnancy screening for research differs from urine pregnancy testing performed for clinical care
- Demonstrate competency with the Quidel Urine Pregnancy kit by performing one positive and one negative result and documenting the results on the hCG-Results QC log sheet
OESO Training: click here for website
- Bloodborne Pathogens Training
- Biosafety Level 2 and BBP for Lab Workers
- Shipping Biological Materials
- Chemical Safety-Orientation
- Chemical Safety-Update
- Laboratory Safety General
Engagement Activity Packet: Study Documentation
(full list of packets is available on Onboarding Learning Plan page)
Study Documentation Regulations and Best Practices - DOCR | 00137344
This course outlines the required components of study documentation for all clinical research, defines standard documentation terminology, and applies knowledge of documentation best practices to everyday scenarios faced by study teams.
Course Objectives:
- Outline required components of study documentation for all clinical research
- Distinguish between documentation regulations and best practice
- Define standard documentation terminology
- Apply knowledge of documentation best practices to day-to-day documentation scenarios faced by study teams
Study Documentation Refresher Series 2: Study Level Documentation | 00145562
This 20 to 25 minute module discusses what study level documentation is, how to maintain it, and resources for creating and maintaining a compliant regulatory binder. You’ll also learn what documents are required for different types of studies and the importance of SOPs.
Study Documentation Refresher Series 4: How To’s and Best Practices | 00145568
This 10 to 15 minute module discussed best practices for maintaining study documentation, proper practices for notation of changes, and when a note/memo to file might be required. You’ll also hear about when a protocol deviation would require both a Note/Memo to File and reporting to the IRB.
eReg Training
eReg is an electronic regulatory binder that interfaces with OnCore. Clinical research staff complete training based on functions they will perform in eREG.
Online training provided by the Society of Clinical Research Sites (SCRS)
- Essential Documents for a Clinical Study: Describes the requirements for Clinical Study essential documents. Describes the type of essential documents collected for a Clinical Study during the different study phases.
- Source Documentation: A review of the terms source data and source documents. Describes the key attributes of source documents, the intent of ALCOA and CCEA and describes the appropriate processes for creating, maintaining and storing source documents.
- Delegation and Training: An overview of study task delegation and the importance of providing/documents study training for site staff.
Conducting a Study - Provided by the Society of Clinical Research Sites
Explains the three stages of study conduct phases including the subject enrollment/recruitment period, the ongoing maintenance and the subject exit/completion phase including the tasks and activities associated with these stages.
Society of Clinical Research Sites
Subject Visit Tracking in OnCore | 00147730
This micro-learning video will (1) provide updated instructions for completion of the Subject Visit Update Screen. (2) help you understand the impact of subject visit tracking on Oncore Financials. (3) Review best practices and their importance in avoiding critical billing errors.
Timeline, Research Ordering, and Essential Linking in Maestro Care
This course reviews workflows for adding timelines, research associating orders and linking appointments & encounters in Maestro Care.
OnCore Training: PC Console Overview for OnCore Financials -DOCR | 00120785
This course is required to be completed as a prerequisite before attending the instructor-lead OnCore Financial Training classes. By the end of this course you will have an introductory understanding of the PC Console for OnCore Financials.
OnCore Training for the Clinical Research Coordinator - DOCR | 00110056
This online training course will provide step by step instructions on completing specific tasks relevant to the basic function(s) of a Clinical Research Coordinator at Duke.
This module covers:
- Subject Administration
- Subject Calendars & Tracking
- Reporting
OnCore Training for the Regulatory Coordinator - DOCR | 00114396
This online training course will provide step by step instructions on completing specific tasks relevant to the basic function(s) of a Clinical Research Regulatory Coordinator at Duke.
This module covers:
- Regulatory Workflows at Duke
- Using the PC Console
- Reporting Functions
Getting Started with Your Clinical Research Portfolio - DOCR | 00141253
This course is part of the Express Start onboarding collection. It will provide tips and tricks for becoming familiar with your clinical research portfolio. This course will also provide tools and resources to help you get started participating in the management of your clinical research studies.
DOCR RPN+ Foundational Series Session recording on Typical Clinical Research Meetings and Participating Effectively.
LinkedIn Learning Videos:
- Run Effective Meetings (3 min)
- Better Meetings (4 min)
Resources:
Research Program Leader Express Start Transcript: Leading Effective Project Meetings

- Ethics and the safety of the people who participate in research is of paramount importance.
- Conducting and documenting informed consent for participants in studies. Developing consent plans and documents for participants in a variety of studies.
- Assisting with the development and submission documentation and information for Institutional Review Board (IRB).
- Collaborating with the research team and the principal investigator to identify adverse events (AEs), and determining whether or not they are reportable.
Engagement Activity Packet: Adverse Events
(full list of packets is available on Onboarding Learning Plan page)
Adverse Events: Identify, Document, Report - DOCR| 00147847 | Web-based offering
The course is designed to help clinical research study teams understand adverse events and the processes surrounding them at Duke. The module addresses identifying adverse events for research studies, collecting and documenting data regarding adverse events, and reporting these events to different governing bodies.
The course provides guidance to help develop a baseline in the following WE-R clinical research competencies:
- Safety and Ethics: Adverse Events
- Safety and Ethics: Sponsor/Regulatory Reporting
Prompt Reporting to the IRB WBT - DOCR | 00145900 | Web-based offering
This 10 minute online module will walk you through protocol deviations and their counterparts protocol violations, adverse events and unanticipated problems, and the subsequent steps of reporting these events to the IRB.
Course Objectives:
- Recognize what constitutes a protocol deviation, violation, adverse event, and unanticipated problem
- Recognize what events need to be reported to the IRB
- Identify FDAAA required informed consent language
- IRecall the reporting timeframe for these events
- Attest to abiding by the policy requirements detailed in Problems or Events that Require Prompt Reporting to the IRB.
CITI Training
- Detecting and Evaluating Adverse Events
- Reporting Serious Adverse Events
These modules are included in the required CITI Good Clinical Practice course. If you have already taken CITI and want to find these modules: log into your Duke Health CITI profile at https://about.citiprogram.org/ and click “View Courses” next to Duke Health. Then click “Review Course” next to the CITI Good Clinical Practice course. Read more about CITI training requirements here.
Adverse Events and Safety (Provided by the Society of Clinical Research Sites)
Explains and defines an Adverse Event. Describes the Investigator’s role and responsibilities regarding Subject Safety and reporting requirements for Serious Adverse Events.
Engagement Activity Packet: Informed Consent
(full list of packets is available on Onboarding Learning Plan page)
Informed Consent Process and Procedures for Clinical Research | 00150119
This course is intended for clinical research study teams and will help you understand planning for informed consent, the informed consent process, and documenting consent at Duke. Note: this course is required by IRB Policy for anyone who will consent participants to a research study.
Learning Objectives:
- Identify the purpose of informed consent and informed consent documentation
- Identify and define types of consent and the elements of the informed consent form (ICF)
- List and describe supporting documentation of the informed consent process (e.g. consent forms, consent notes, waivers).
- Identify and interpret policies and regulations related to the informed consent process and HIPAA
- Describe requirements related to ensuring participant comprehension of informed consent and ICFs (e.g. reading level and translation requirements)
- Describe how the consent process is planned and diagnose errors in consent planning
- Describe the procedure for conducting and documenting consent for participants
Elements of Effective eConsent Design | 00163541
This module reviews the elements of effective eConsent design and delivery to support effective informed consent to clinical research. Upon completion of this online module, you will be able to describe these elements, including: cognitive load, multimedia, interactivity, and user-centered design. This module is system agnostic, so it is not specific to using REDCap to create an eConsent. Rather, it covers how to use what we know about learning to develop an eConsent that enhances participant understanding.
Learning Objectives:
- Define eConsent
- Discuss basic concepts of cognitive load
- Discuss basic concepts of multimedia theory
- Define interactivity
- Discuss components of user-centered design
Informed Consent: Remote Consenting - DOCR | 00147618
This course is intended for study team members at Duke who may consent participants to clinical research studies remotely. Remote means the consent process is not occurring in-person.
ConsentTools.org - This site was developed by the WUSTL School of Medicine.
- Assessments – This section includes a validated assessment instrument for assessing participant understanding of a study during the informed consent process. There are several videos on when to assess understanding of consent information, how to administer an assessment, and what to do with assessment results.
- LARs – This section includes tools for discussing the need for an LAR and videos on how to determine the need for an LAR and how to help participants appoint an LAR.
- eConsent – Describes evidence-informed practices when designing their eConsent processes.
MC Clinical Research 100 E-Learning Module | 00129623
This module provides instruction for new clinical researchers who will work in a Clinical Research Coordinator (CRC) user role or as a refresher for existing clinical research staff. Research Dashboard and system functionality will be reviewed in the module. This course, as well as passing an end-of-course test, is a requirement for CRC access to Maestro Care.
This module covers:
- The Research Dashboard
- Enrolling Patients in Studies
- Order Management
- Encounters-linking
- Conducting and Documenting a Patients Study Visit
- Navigating the In Patient Record- Chart Review
CITI Training
- Informed Consent in Clinical Trials of Drugs, Biologics, and Devices
This module is included in the required CITI Good Clinical Practice course. If you have already taken CITI and want to find these modules: log into your Duke Health CITI profile at https://about.citiprogram.org/ and click “View Courses” next to Duke Health. Then click “Review Course” next to the CITI Good Clinical Practice course. Read more about CITI training requirements here.
Online Training Provided by the Society of Clinical Research Sites (SCRS)
- Clinical Practice vs. Clinical Research: An overview of the differences between the activities which occur when managing patients during routine Clinical Practice versus the activities when you are managing subjects as part of a Clinical Research Study.
- IRB/IEC Responsibilities and Informed Consent: Overview of the purpose and activities of the IRB/IEC and the Investigator’s responsibilities in accordance with ICH-GCP. Describes the process of informed consent and the responsibilities of the Investigator when obtaining informed consent.
Developing the Informed Consent Form | DOCR 00150391
This module is designed to provide study teams with fundamental or refresher information on developing the informed consent form. Upon completion you will be able to develop the informed consent form to meet Duke Health IRB and federal standards.
Course Objectives:
- Discuss the purpose of informed consent and of documentation
- Identify and define the elements of the informed consent form
- Identify and interpret policies and regulations related to the informed consent process and Health Insurance Portability and Accountability Act (HIPAA)
- Describe requirements related to ensuring participant comprehension of informed consent and ICFs (e.g., reading level and translation requirements)
Elements of Effective eConsent Design | 00163541
This module reviews the elements of effective eConsent design and delivery to support effective informed consent to clinical research. Upon completion of this online module, you will be able to describe these elements, including: cognitive load, multimedia, interactivity, and user-centered design. This module is system agnostic, so it is not specific to using REDCap to create an eConsent. Rather, it covers how to use what we know about learning to develop an eConsent that enhances participant understanding.
Learning Objectives:
- Define eConsent
- Discuss basic concepts of cognitive load
- Discuss basic concepts of multimedia theory
- Define interactivity
- Discuss components of user-centered design
REDCap eConsent and Multi-Language | DOCR-RED-560 | Live Class
This live class includes an overview of how to create an eConsent using REDCap and how to use Multi-Language features in REDCap.
ConsentTools.org - This site was developed by the WUSTL School of Medicine.
- Optimizing Key Information – This section includes videos, examples, and sample language to optimize key study information to facilitate participant understanding.
CITI Training
- Informed Consent in Clinical Trials of Drugs, Biologics, and Devices
This module is included in the required CITI Good Clinical Practice course. If you have already taken CITI and want to find these modules: log into your Duke Health CITI profile at https://about.citiprogram.org/ and click “View Courses” next to Duke Health. Then click “Review Course” next to the CITI Good Clinical Practice course. Read more about CITI training requirements here.
Clinical Practice vs Clinical Research (Provided by the Society of Clinical Research Sites)
An overview of the differences between the activities which occur when managing patients during routine Clinical Practice versus the activities when you are managing subjects as part of a Clinical Research Study.
IRB Overview - DOCR-RES-300
This course offers an overview of the DUHS IRB review process and requirements, federal regulations, and Duke policies pertaining to research involving human subjects. The course highlights tools available to study teams on the IRB web site; presents an example of an eIRB submission; and provides strategies for working efficiently with the IRB.
Course Objectives:
- Describe the DUHS IRB’s scope of oversight – what does and what does not require review by the DUHS IRB
- Describe the IRB review process
- Outline a basic overview of the eIRB system, including how to gain access to eIRB, how to use and navigate within eIRB, how to find out study status, and how to get help with eIRB
- Describe reporting to the IRB
- Describe how to communicate with the IRB and make use of the many tools available on the IRB website
iRIS Training
The iRIS system is used to IRB application submissions and maintenance. Training is not required to access iRIS but can be helpful depending on your involvement with managing existing protocol applications or submitting protocols to the Duke Health IRB.
Research Ethics in the Context of Clinical Research - TRECC | 00152210
This course explains the basics of ethical principles in clinical research and the unethical history that transformed the way clinical research was conducted. This presentation has three objectives:(1) Recognize why it is important to adhere to ethical principles while conducting clinical research (2) Describe historical unethical research studies that led to the establishment of ethical guidelines and regulations governing clinical research (3) Describe the main ethics principles in the context of human subjects research
CITI Training
- Vulnerable Subjects - Research Involving Prisoners
- Vulnerable Subjects - Research Involving Children
- Vulnerable Subjects - Research Involving Pregnant Women, Fetuses, and Neonates
These modules are included in the required CITI Vulnerable Subjects courses. If you have already taken CITI and want to find these modules: log into your Duke Health CITI profile at https://about.citiprogram.org/ and click “View Courses” next to Duke Health. Then click “Review Course” next to the appropriate course in CITI. Read more about CITI training requirements here.
IRB/IEC Responsibilities and Informed Consent (Provided by the Society of Clinical Research Sites)
Overview of the purpose and activities of the IRB/IEC and the Investigator’s responsibilities in accordance with ICH-GCP. Describes the process of informed consent and the responsibilities of the Investigator when obtaining informed consent.
ClinicalTrials.gov Online Modules
| Course Name and Description | Link |
|---|---|
|
Intro to ClinicalTrials.gov: Regulation and Policy Overview: This module will provide an introduction to ClinicalTrials.gov and cover why it is important to register and report results, who is responsible, when to register, and the Duke process. |
Register |
| ClinicalTrials.gov: Registering Trials and Maintaining Records: This module will walk you through who can register trials in ClinicalTrials.gov, what information is included in a registration, resources to help with registration, and what is required for record maintenance once a trial is registered. | Register |
| ClinicalTrials.gov: Results Submission Overview: This module will cover which studies must submit results, what information is required for results submission, when to submit results, and Duke's process for results submission. | Register |
DOCR ClinicalTrials.gov Microlearning Video Stream Channel
Study Documentation Regulations and Best Practices - DOCR | 00137344
This course outlines the required components of study documentation for all clinical research, defines standard documentation terminology, and applies knowledge of documentation best practices to everyday scenarios faced by study teams.
Course Objectives:
- Outline required components of study documentation for all clinical research
- Distinguish between documentation regulations and best practice
- Define standard documentation terminology
- Apply knowledge of documentation best practices to day-to-day documentation scenarios faced by study teams
Study Documentation Refresher Series 1: Introduction to Clinical Research Documentation | 00145375
This 12 to 15-minute module discusses the importance of study documentation and how to navigate different governing authorities as it relates to study documentation.
Prompt Reporting to the IRB WBT - DOCR | 00145900
This 10 minute online module will walk you through protocol deviations and their counterparts protocol violations, adverse events and unanticipated problems, and the subsequent steps of reporting these events to the IRB.
Course Objectives:
- Recognize what constitutes a protocol deviation, violation, adverse event, and unanticipated problem
- Recognize what events need to be reported to the IRB
- Identify FDAAA required informed consent language
- IRecall the reporting timeframe for these events
- Attest to abiding by the policy requirements detailed in Problems or Events that Require Prompt Reporting to the IRB.
Investigational New Drug Sponsor and Investigator Responsibilities | 00122655
This training will cover the responsibilities associated with maintaining an IND and is intended for academic investigators who will hold an IND. This training consists of ten modules. The first nine modules will cover IND sponsor responsibilities that must be fulfilled by investigators who conduct a clinical investigation run under an IND. Investigators fulfilling a dual role as sponsor and investigator, in other words, sponsor-investigators, must fulfill the responsibilities of both sponsors and investigators.
The Modules include:
- Introduction to IND Sponsors and Investigator Responsibilities
- Overview of IND Maintenance and Tracking
- IND Annual Reports
- IND Safety Reporting
- IND Information and Amendments
- Maintaining Accountability Records for INDs
- IND Protocol Amendments
- IND Monitoring and Multi-Center Trails
- Financial Disclosure
- IND Investigator Responsibilities
Investigational Device Exemption Sponsor and Investigator Responsibilities | 00122525
This training will cover the responsibilities associated with maintaining an IDE and is intended for academic investigators who will hold that IDE for a significant risk device study. This training consists of ten modules. The first nine modules will cover IDE sponsor responsibilities and the final module will cover responsibilities that must be fulfilled by investigators who conduct a significant risk device study run under an IDE. Investigators fulfilling a dual role as sponsor and investigator, in other words, sponsor-investigators, must fulfill the responsibilities of both sponsors and investigators.
The Modules include:
- Introduction to IDE Sponsor and Investigator Responsibilities
- Overview of IDE Maintenance and Tracking
- IDE Progress Reports and Final Reports
- IDE Modifications
- Unanticipated Adverse Device Effects
- IDE Reports
- Maintaining Accountability Records for IDEs
- IDE Monitoring and Multi-Center Trails
- Financial Disclosure
- IDE Investigator Responsibilities

- Protecting participant study data is vitally important.
- Safeguarding the participant data that is collected and entered (whether on paper or via an electronic system). Ensuring accuracy and completeness of data for all the studies.
- Developing data collection and/or entry plans, SOPs, or tools.
- Developing or reviewing Duke Research Data Lifecycle (DRDL) plans that outline how the study's research data will be managed and protected throughout the study.
Knowledge Objectives
- Define key principles of accurate and efficient data collection and entry in clinical research.
- Apply appropriate tools (e.g., Excel, REDCap, Qualtrics) to develop data entry systems aligned with study workflows.
- Recognize strategies to design data collection systems that reduce respondent burden and ensure data accuracy.
- Describe the importance of review and testing to evaluate quality and usability of data collection tools.
- Train others in the use and purpose of data collection protocols and systems as appropriate.
Learning Resources
- Duke LMS - Fundamentals of Clinical Research Data Management & Collection - DOCR | 00163171
- REDCap Training Series | Found on DOCR Course List under “Upcoming Live DOCR Course Schedule”
- REDCap application: Training Videos
- myRESEARCHpath: Devising the Data Strategy
- Coursera: Clinical Data Management by Vanderbilt
Knowledge Objectives
- Describe the importance of data accuracy, completeness, and protocol adherence in clinical research.
- Apply standard quality control checks to identify errors or inconsistencies in study data.
- Develop tools or templates to streamline data quality assurance procedures.
- Identify and interpret trends or recurring issues in data quality and describe appropriate escalation procedures.
- Implement corrective actions or training plans in response to identified data quality concerns.
Learning Resources
- myRESEARCHpath: Research Quality and Reproducibility Online Course
- Duke LMS: Study Documentation Regulations and Best Practices – DOCR | 00137344
- Coursera: Clinical Trials Data Management and Quality Assurance by Johns Hopkins
The Duke Biostatistics, Epidemiology, and Research Design (BERD) Methods Core Biostats Resources for Non-Statisticians provides training and resources on clinical research and data analysis. Review their training videos on Intro to Clinical Research and Data Analysis.
Fundamentals of Clinical Research Data Management & Collection | 00163171
This course will help clinical research study teams identify the goals of research data management and summarize approaches for keeping data secure and avoiding errors. The learner will be able to define the reason for data management, describe best practices for storing and sharing data to ensure data integrity and security, define data collection methodology, describe database design best practices, and discuss tips and tricks for collecting data for research purposes.
CITI Training
- Reproducibility of Research Results
This module is included in the required CITI Good Clinical Practice course. If you have already taken CITI and want to find these modules: log into your Duke Health CITI profile at https://about.citiprogram.org/ and click “View Courses” next to Duke Health. Then click “Review Course” next to the CITI Good Clinical Practice course. Read more about CITI training requirements here.
OESO Training
Knowledge Objectives
- Describe best practices for storing data
- Identify and interpret Duke’s policies regarding data ownership and data security
- Identify best practices for keeping data secure
- Describe the purpose of data security and identify key terms
- Identifying approved systems for data security and how to utilize them
Learning Resources
- Annual HIPAA Privacy and Security Training | 00156846
- Annual Security Awareness Training | 00152160
- Duke LMS: Information Security for Research Staff | DOCR-RES-520 | Live Class
- Duke LMS: PACE Training for Duke Employees and Affiliates | 00131237
- Duke Information Security Office: Policies, Procedures, and Standards for Data Security and Security Guides, including Duke Services, Data Classification Standard, and Secure Collection of SSNs
- MyRESEARCHpath: Determine Compute and Data Storage Solutions
- Duke Office of Audit, Risk, and Compliance: Privacy
Engagement Activity Packet: Data Security and Provenance
(full list of packets is available on Onboarding Learning Plan page)
Fundamentals of Clinical Research Data Management & Collection - DOCR | DOCR-DATAMGMT-101
This course will help clinical research study teams identify the goals of research data management and summarize approaches for keeping data secure and avoiding errors. The learner will be able to define the reason for data management, describe best practices for storing and sharing data to ensure data integrity and security, define data collection methodology, describe database design best practices, and discuss tips and tricks for collecting data for research purposes.
NIH Data Sharing Trainings
NIH Scientific Data Sharing: About | Data Sharing (nih.gov)
Links to trainings on the requirements and what type of research should be sharing data: Learning | Data Sharing (nih.gov)
OESO Training
Annual HIPAA Privacy and Security Training
Adobe Sign Training for Clinical Research - DOCR | 00147697
This training is intended for clinical research personnel at Duke who will request access to Adobe Sign from the Duke Office of Clinical Research. Course Objectives: 1) Define Adobe Sign. 2) Describe how you can and cannot use Adobe Sign. 3) Differentiate between e-signatures and digital signatures. 4) Find tools and resources.
Knowledge Objectives
- Identify the benefits and barriers to data sharing in research
- Recognize the appropriate use of data repositories in different contexts
- Understand Institutional and NIH guidelines and requirements for data sharing
- Apply best practices for data sharing
- Demonstrate knowledge of compliance and ethical considerations in data sharing
Learning Resources
- myRESEARCHpath: Guidance for Sharing Research Data
- myRESEARCHpath: Engage in Open Science and Open Scholarship
- myRESEARCHpath: NIH Data Management and Sharing Policy
- Duke Center for Data and Visualization Sciences: Sharing Participant Data Video
- Duke Faculty Handbook: Research Data Ownership
- myRESEARCHpath: Duke Data Front Door
Knowledge Objectives
- Define key terms and concepts related to data standards in clinical research.
- Recognize the purpose and benefits of using Common Data Elements (CDEs) and standardized data in research.
- Identify and apply the FAIR data principles in clinical research.
- Distinguish between different types of health data standards and their appropriate use cases.
- Recognize the role of regulatory bodies and global standards organizations in clinical data management.
Learning Resources
- NIH/National Library of Medicine Courses:
- Support: Duke Libraries FAIR Data Resources
- CDISC: Data Standards
Knowledge Objectives
- Identify opportunities to enhance research processes using innovative technologies.
- Adapt existing tools to improve workflow or data quality.
- Use innovative tools to streamline research operations or enhance efficiency.
- Recognize the process for approval to use new software or technology for research.
- Troubleshoot issues in the application of new technologies in research settings.
- Train team members on how to effectively use new or adapted technologies in their research work as appropriate.
Learning Resources
REDCap: Building in the Data Dictionary | DOCR-RES-560 | In-Person
Learn to use a Microsoft Excel file to build or modify the structure of your database in a concise format. The data dictionary is the preferred method of creating your database. This method makes it easy and quick to create many variables, and use advanced functionality (branching logic or calculated fields).
Knowledge Objectives
- Identify data elements from a protocol
- Identify data that contains PHI or restricted information
- Describe appropriate modes of electronic data transmission
- Describe appropriate modes of data storage
- Identify data elements residing within the Duke covered entity and those being transmitted outside of it
Learning Resources
- myRESEARCHpath: Determine Compute and Data Storage Solutions
- myRESEARCHpath: Develop the Data Management Plan
- myRESEARCHpath: Duke Research Data Lifecycle (DRDL) Plan Guidance
NIH Training: Data Management Plans
REDCap Workshop: Start Building - Part 1 | DOCR-RES-530-P1 | In-Person
In this workshop learners will create a project using REDCap.
Course Objectives:
- Practice building in REDCap, including application of field types, validation, and branching
- Identify REDCap features and determine how to apply these features on a range of projects
- Summarize the steps for moving projects to production
- Identify best practices for collecting data
Completion of the Research Database Design Principles course is a pre-requisite for this course.
REDCap Workshop: Start Building - Part 2 | DOCR-RES-530-P2 | In-Person
In this workshop learners will create a project using REDCap.
Course Objectives:
- Practice building in REDCap, including application of field types, validation, and branching
- Identify REDCap features and determine how to apply these features on a range of projects
- Summarize the steps for moving projects to production
- Identify best practices for collecting data
Completion of the Research Database Design Principles course is a pre-requisite for this course.
CITI Training
- Data Management (RCR Basic)
These modules are included in the CITI RCR Basic course. If you have already taken RCR through CITI and want to find these modules: log into your Duke RCR CITI profile at https://about.citiprogram.org/ and click “View Courses” next to Duke RCR. Then click “Review Course” next to the RCR course. Read more about CITI training requirements here.
Knowledge Objectives
- Define key concepts and terminology related to data management and cleaning.
- Apply best practices for data cleaning and file management.
- Identify and describe institutional data management policies and procedures.
- Recognize and describe the components and development of a data management plan.
- Describe the purpose of data management.
Learning Resources
- myRESEARCHpath: Develop the Data Management Plan
- Harvard Open Science Framework: Research Data Management Lifecycle Checklist
- Coursera: Best Data Cleaning Courses – Includes Excel, Python, and R-based training
- NIH Training: Data Management Plans
Knowledge Objectives
- Define key terms and concepts related to de-identification
- Identify methods and procedures for de-identification
- Differentiate between de-identified data, limited data sets, and PHI
- Recognize HIPAA identifiers and how to handle data in compliance with HIPAA regulations
- Ensure compliance with federal and Institutional policies regarding de-identification
Learning Resources
- Duke Health IRB Policy: De-Identification of PHI
- Duke HR: Protected Health Information and Patient Privacy Policy
- Annual HIPAA Privacy and Security Training | 00156846
Knowledge Objectives
- Differentiate between rapid and in-depth qualitative analysis methods.
- Use qualitative data analysis software (e.g., NVivo, Atlas.ti) to code, categorize, and query data.
- Develop coding schemes using both pre-set frameworks and inductive methods.
- Interpret and synthesize qualitative findings in alignment with study objectives.
- Collaborate with team members to integrate qualitative findings into research products such as manuscripts or reports.
Learning Resources
- myRESEARCHpath: Design the Analysis Plan
- Duke Medical Center Library: Qualitative Research Guides
- Duke University Libraries: Qualitative Research – Getting Started
- ATLAS.ti: Video Tutorials and ATLAS.ti Academy
- Duke University Libraries: Introduction to NVivo
- Duke Department of Population Health Sciences – Qualitative Research Partners (QualCore)
Knowledge Objectives
- Use statistical software (e.g., SPSS, SAS, R) to import, clean, and code quantitative data for analysis.
- Conduct descriptive analyses to summarize study data (e.g., frequencies, means, medians).
- Identify appropriate statistical tests based on research questions and data type.
- Perform inferential statistical tests (e.g., t-tests, chi-square) to explore relationships in data.
- Collaborate with statisticians and investigators to develop and execute statistical analysis plans and interpret results for deliverables.
Learning Resources
- myRESEARCHpath: Design the Analysis Plan
- Duke Pathways: Introduction to Data Science with R Pathway
- Duke BERD Core: Guidance for the Implementation of Quantitative Best Practices in Healthcare Research
- Duke Medical Center Library Guides:Data Analytics and Visualization Software
- Duke University Libraries: Statistical Science Software, Tools, and Tutorials
- Coursera: Clinical Data Management by Vanderbilt – Includes basic quantitative data handling.
- Coursera: Data Analysis with R Specialization by Duke
- Program: Data Analytics Certificate – Duke Continuing Studies
- Describe the ethical and regulatory considerations in returning results to study participants.
- Draft clear and accessible lay summaries of research results for participant audiences.
- Create participant-facing materials (e.g., tables, fliers, visuals) that accurately and sensitively present study findings.
- Apply readability and health literacy tools to evaluate the accessibility of return-of-results materials.
- Describe how to implement a dissemination plan that aligns with study goals and participant needs.
Learning Resources
- MyRESEARCHpath: Return of Results to Participants
- MyRESEARCHpath: Engage in Open Science and Open Scholarship
- LMS: Readability Fundamentals for Clinical Research Participant Engagement Materials | 00160856
- Identify key performance indicators and metrics for tracking clinical study progress.
- Create effective reports and visualizations that communicate study progress and milestones.
- Analyze study data to identify emerging trends or issues affecting performance.
- Identify potential study issues and formulate a response or escalation plan.
- Train colleagues on best practices for developing and interpreting study metrics and dashboards as appropriate.
Learning Resources
- myRESEARCHpath: Manage Study and Maintain Institutional Approval
- Duke Libraries: Data Visualization Workshops
- Duke Libraries: Center for Data and Visualization Sciences
Knowledge Objectives
- Recognizing the purpose and significance of validation and testing scripts for EDC system.
- Defining terms related to field validation and testing.
- Adhering to regulatory compliance requirements for 21 CFR Part 11.
- Execute the validation and testing processes for EDC systems.
- Recognizing common practices for proper documentation and reporting of testing and validation activities.
Learning Resources
- FDA: Electronic Systems, Electronic Records, and Electronic Signatures Webinar
- REDCap: eConsent Guidelines
- REDCap: Training Videos
- DOCR Guidance: 21 CFR Part 11
- LMS: Elements of Effective eConsent Design | 00163541

- Understanding research study designs to contribute where needed.
- Assisting with the development of grant applications or funding proposals for sponsored studies. Using those proposals to work with investigators in the development of study designs and research study protocols.
- Helping to develop scientific publications or presentations. Conducting literature searches and reviews.
Budget and Payment Terms Basics for Industry-Sponsored Clinical Trials | 00152966
This course is designed to help study teams understand the basics of budgeting and payment terms for industry-sponsored clinical trials/research. Learning Objectives: Upon completion of this module, you will be able to: Identify the study documents to review when developing a budget, Describe the types of costs associated with conducting a study, Recognize CRU Management Fees and Duke’s Facilities & Administrative Costs (F&A), Discuss tips for negotiation, Discuss common negotiation mistakes, Recall preferred payment terms.
Introductory Overview of Sponsored Research - DOCR/OPSD | 00146886
This course will provide individuals engaged in clinical and biomedical research with an introductory overview of sponsored research. Those interested in learning more about sponsored research in preparation for identifying a research strategy and funding opportunities within their research discipline should check it out. This course was created in collaboration between DOCR and the Office of Physician Scientist Development for the Pediatric Scientist Development Program.
Getting Started with Research Funding - DOCR/OPSD | 00146887
This course will provide individuals engaged in clinical and biomedical research with introductory information about federal, and non-federal, research funding resources and tips for navigating research funding opportunities that align with their research objectives. This course was created in collaboration between DOCR and the Office of Physician Scientist Development for the Pediatric Scientist Development Program.
Early Career Funding Opportunities Overview - DOCR/OPSD | 00146888
This course will provide guidance on NIH-sponsored Training, Fellowship, and Career Development award programs. Including a discussion on how these programs relate to a larger picture of career progression. While this course will mainly focus on the NIH; the NSF, DOD, and foundations are also covered lightly. This course was created in collaboration between DOCR and the Office of Physician Scientist Development for the Pediatric Scientist Development Program.
Financial Basics for Clinical Research - DOCR/RCC | 00140600
Participants will gain a broad understanding of the financial concepts associated with the life cycle of a clinical research study. Learning Objectives: 1) Outline the purpose and use of an internal cost assessment, how it impacts the negotiation of the study budget, and why payment terms within a contract are important. 2) Discuss the concept of revenue management, specifically how the work performed and milestones outlined in the study protocol determine earned revenue. 3) Identify the type of costs incurred as the work is performed on a study and the concept of effort management. 4) Outline the use and importance of financial reporting for CRU management and the principal investigator.
The Duke Medical Center Library and Archives offers training classes, consultations, and resources on scholarly communications and tools for literature reviews.
LinkedIn Learning Videos:
- How to Develop a Literature Review (3 min)
- Purpose of a Literature Review (2.45 min)
- How to Develop a Literature Review (3min)
- Tools for Reviewing the Scientific Literature (4min)
Introduction to Submitting an IRB Application in iRIS - DOCR | 00133297
This online course is designed to outline the basic process for IRB application submission using the iRIS system. The module will describe the workflow among the two main research workflow systems iRIS and OnCore and will introduce users to the iRIS system, basic navigation, and initial protocol submission.
Clinical Research Overview - Provided by the Society of Clinical Research Sites (SCRS)
Introduces and describes the phases of a Clinical Research Study and the various study designs.
Duke Biostatistics, Epidemiology, and Research Design (BERD) Methods Core: Online Modules for Non-Statisticians
A series of online training videos addressing the following topics:
- Introduction to Research and Design
- Formulating the Research Question
- The Null and Alternative Hypothesis
- Study Design and Data Collection
CITI Training
- Intro to RCR
These modules are included in the CITI RCR Basic course. If you have already taken RCR through CITI and want to find these modules: log into your Duke RCR CITI profile at https://about.citiprogram.org/ and click “View Courses” next to Duke RCR. Then click “Review Course” next to the RCR course. Read more about RCR training requirements here.
Society of Clinical Research Sites (SCRS) Training:
Clinical Research Overview: Introduces and describes the phases of a Clinical Research Study and the various study designs.
Conducting a Study: Explains the three stages of study conduct phases including the subject enrollment/recruitment period, the ongoing maintenance, and the subject exit/completion phase including the tasks and activities associated with these stages.
The Duke Medical Center Library and Archives offers training classes, consultations, and resources on scholarly works.
CITI Training
- Reproducibility of Research Results
These modules are included in the CITI RCR Basic course. If you have already taken RCR through CITI and want to find these modules: log into your Duke RCR CITI profile at https://about.citiprogram.org/ and click “View Courses” next to Duke RCR. Then click “Review Course” next to the RCR course. Read more about RCR training requirements here.
LinkedIn Learning Videos:

- Managing individual studies, or the institution as a site for larger studies. Understanding the details of the research studies and protocols. Gathering information to help determine if participation is feasible.
- Preparing for, coordinating, and actively participating in site visits (study initiation, monitoring, audits) and regularly communicating with study sponsors.
- Ensuring that protocols are conducted in compliance with institutional requirements and other policies. Applying regulations, policies, and procedures, and knowing how to troubleshoot when questions arise.
- Managing participants and protocols in the Duke Clinical Research Management System (OnCore).
Research Professionals Network+ Session Recording: Typical Clinical Research Meetings and Participating Effectively
Monitoring and Auditing (Provided by the Society of Clinical Research Sites)
Describes the routine monitoring and auditing activities that occur during a clinical study. This topic includes information on the purpose, what to expect, and tips to prepare for these Sponsor activities.
DOCR OnCore Microlearning WarpWire Channel
Maestro Care for Clinical Research Training
Maestro Care training is available for clinical research professionals by function and role. Specific training is required depending on access needed to the Electronic Medical Record in Maestro Care (Epic). Visit the MC page and review training based on functional need/role.
OnCore and eREG Training
OnCore is the Clinical Research Management System for clinical research activities at Duke. eReg is an electronic regulatory management system used by some study teams at Duke to store regulatory documents. You should complete training based on functions you will perform and access needed in OnCore and eReg. Available training is listed on the support page.
Duke ClinCard Study Coordinator/CRC Learning Module | 00184995
This training is for those identified by their department as Study Coordinators (SCs) or Clinical Research Coordinators (CRCs) in the Duke ClinCard System, who use the system for participant reimbursement for clinical research studies. This training must be completed before Employee Travel and Reimbursement will grant access to the Duke ClinCard System.
Express Start Onboarding Program-CRC | 00141671
The Express Start Onboarding Program consists of four self-paced e-learning courses that will provide an overview of clinical research activities, regulations, and workflows at Duke.
Learning Objectives:
- Describe the roles and responsibilities of a Clinical Research Coordinator
- Identify the clinical research offices and resources within Duke Health
- Identify the clinical research systems used at Duke and understand how they interact with one another
- Discuss the studies that make up your research portfolio
- Describe the career progression within clinical research professions
iRIS Training
The iRIS system is used to IRB application submissions and maintenance. Training is not required to access iRIS but can be helpful depending on your involvement with managing existing protocol applications or submitting protocols to the Duke Health IRB.
OnCore Training for the Regulatory Coordinator - DOCR | DOCR-ONCR-150
This online training course will provide step-by-step instructions on completing specific tasks relevant to the basic function(s) of a Clinical Research Regulatory Coordinator at Duke. This module covers:
- Regulatory Workflows at Duke
- Using the PC Console
- Reporting Functions
OnCore Administrative Leadership Essentials - DOCR | DOCR-ONCR-160
This online training course is intended for administrative leadership in Duke School of Medicine who are granted organizational access in the OnCore system (Dean, Assistant Dean, Chairmen, Business Managers, other central office staff). This designated role in OnCore will be able to view protocols in the PC Console, view accrual and enrollment information on clinical research studies, and generate and schedule administrative reports for departments and units at Duke.
This module covers:
- Administrative Leadership Overview & Using the PC Console
- Using the Accrual Monitoring Console
- Reporting Functions
Express Start Onboarding Program-Regulatory Coordinator | 00145771
The Express Start Onboarding Program consists of four self-paced e-learning courses that will provide an overview of clinical research activities, regulations, and workflows at Duke.
Learning Objectives:
- Describe the roles and responsibilities of a Regulatory Coordinator
- Identify the clinical research offices and resources within Duke Health
- Identify the clinical research systems used at Duke and understand how they interact with one another; with a spotlight on iRIS
- Find and follow applicable standards, guidelines, regulations, policies, and procedures for performing clinical research at Duke
- Describe the career progression within clinical research professions
Podcast: An In Depth Look at Clinical Trial Feasibility Surveys from A Sponsor and Site Perspective – Dan Sfera, “Clinical Trials Guru”
LinkedIn Learning Videos:
- Project Management Foundations > Initiate a Project (1.5 min)
- Why Projects Fail > Identify and Align with Organizational Goals and Objectives (3.5 min)
- Project Management Foundations: Small Projects > Define Project Scope (2.5 min)
- Project Management Foundations > Prepare a Project Scope Statement (2.21 min)
- Why Projects Fail and How to Improve Their Success > Avoid Scope Creep (3.5 min)
RPL Onboarding Engagement Activity Packet: Budgeting and Resource Management
RPL Express Start Lesson Transcripts
Duke Procurement Training
(These are a variety of available trainings around procurement that you may not need right now, bookmark and come back to them when you need them)
- Learning to Use Buy@Duke
- So, You Need to Make a Purchase? Procuring Goods and Services at Duke (live virtual class – FIN-AP-101)
- Financial Services Training and Schedules
Duke ClinCard Study Coordinator/CRC Learning Module | 00184995
This training is for those identified by their department as Study Coordinators (SCs) or Clinical Research Coordinators (CRCs) in the Duke ClinCard System, who use the system for participant reimbursement for clinical research studies. This training must be completed before Employee Travel and Reimbursement will grant access to the Duke ClinCard System.
LinkedIn Learning Videos
- Project Management Foundations > How to Plan Procurement (3min)
- Project Management Foundations > Develop a Project Budget (3.5min)
MyRESEARCHpath Resources and Pages
- Develop the Budget and Justification
- Plan and Refine Project (Find Equipment, Resources, and Service Centers)
- Commercial or Industry Sponsored Clinical Trial Budgets
- Manage Award and Finances
TIP: Use the “get help” function on each page to review which offices to go to for help with project budgets!
Budget and Payment Terms Basics for Industry-Sponsored Clinical Trials - DOCR | 00152966
This online course is designed to help study teams understand the basics of budgeting and payment terms for industry-sponsored clinical trials/research. Learning Objectives - Upon completion of this module, you will be able to:
- Identify the study documents to review when developing a budget
- Describe the types of costs associated with conducting a study
- Recognize CRU Management Fees and Duke’s Facilities & Administrative Costs (F&A)
- Discuss tips for negotiation
- Discuss common negotiation mistakes
- Recall preferred payment terms
Financial Basics for Clinical Research | 00155465
This online course is designed to help participants understand the financial concepts associated with the life cycle of a clinical trial/research study.
Course Objectives:
- Describe clinical research from a financial perspective
- Describe the types of costs associated with a clinical trial/research study
- Describe the concept of effort management
- Describe financial and management reporting
- Describe financial close-out requirements
- Describe systems useful in following financial activities associated with a clinical trial/research study
RPL Express Start Lesson Transcript: Project Management Fundamentals > Managing and Reporting Risk
LinkedIn Learning Video
- Project Management Foundations: Risk > The Components of A Project Management Risk Plan (3.3min)
Study Documentation Regulations and Best Practices - DOCR | 00137344
This course outlines the required components of study documentation for all clinical research, defines standard documentation terminology, and applies knowledge of documentation best practices to everyday scenarios faced by study teams.
Course Objectives:
- Outline required components of study documentation for all clinical research
- Distinguish between documentation regulations and best practice
- Define standard documentation terminology
- Apply knowledge of documentation best practices to day-to-day documentation scenarios faced by study teams
Institutional Regulatory Policies and Procedures in Clinical Research Management - DOCR | 00139987
This course will provide Duke employees engaged in clinical research with information about regulations, guidelines, and policies for conducting clinical research at Duke University.
Learning Objectives:
- Find and follow applicable standards, guidelines, regulations, policies, and procedures for performing clinical research at Duke
- Identify and interpret policies and regulations regarding Duke Health IRB oversight of research
- Describe study elements that must be approved by or reported to the Duke Health IRB
- Identify research approval elements and responsible offices at Duke
CITI Training
- Conflicts of Interest
- Research Misconduct (RCR Basic)
These modules are included in the CITI RCR Basic course. If you have already taken RCR through CITI and want to find these modules: log into your Duke RCR CITI profile at https://about.citiprogram.org/ and click “View Courses” next to Duke RCR. Then click “Review Course” next to the RCR course. Read more about RCR training requirements here.
TBD
Engagement Activity Packet: Study Closeout
(full list of packets is available on Onboarding Learning Plan page)
Regulatory Closeout Basics for Clinical Research | DOCR - 00150856
This course will guide clinical research study teams through the basics of regulatory study closeout
Course Objectives:
- Prepare studies for regulatory closeout as dictated by institutional guidelines
- Recall the basics of document storage
Study Documentation Refresher Series 5: Document Retention | 00145569
This 10 to 15-minute module discusses how documents are retained and stored for open and closed studies as well as how regulations inform document retention requirements. You’ll also learn tips on planning for document storage and retention.

- Demonstrating good conduct, forethought, and critical thinking.
- Proactively seeking to expand skills, complete necessary professional certifications, and enhance overall job competencies.
- Keeping current with research updates and practices by attending key internal professional development opportunities (e.g., Research Wednesday, the Research Professionals Network (RPN)) as well as relevant external opportunities (e.g., SoCRA, ACRP, etc.). Applying what was learned in the role and sharing learn new knowledge with your colleagues.
- Anticipating problems before they arise, navigating processes and people involved in clinical research, and demonstrating organizational awareness.
- Exhibiting teamwork and effective interpersonal skills to get work done efficiently and effectively.
Duke Professional Development Resources for Project Staff (Not an Exhaustive List)
- Duke Project Management Community of Practice – Monthly Educational Sessions: Link to Join
- DOCR Research Wednesdays – Monthly Educational Sessions
- DOCR Research Professionals Network – Monthly Educational Sessions
- Review website and identify tools: DOCR Onboarding and Training for Clinical Research Professionals
- Review website and identify tools: CTSI Education and Workforce Development
- Review website: CTSI Events
- Subscribe to the CITI Newsletter for access to free live webinars
- Review website and identify tools: Duke HR L&OD professional development offerings
- Duke Medical Center Library and Archives Training and Consulting
Clinical Research Professional Organizations
External Awareness and Contribution resources will vary greatly depending on scientific area and needs of your team. In the context of clinical research this means keeping current with advances in the scientific area and consider the impact on the clinical research program. Keeping self and team current with research updates by attending external offerings; and applying learned material on the job. Playing a leadership role on committees and workgroups.
Tips:
- Explore internal or external resources or groups (newsletters, journals, social media pages, events, conferences, professional associations, etc.) to subscribe to and/or attend so you are kept up to date with advances in your area.
- Become familiar with the bodies that govern your work and any additional governing bodies you may need to research and learn more about.
Bookmark MyRESEARCHpath and MyRESEARCHhome
This is an excellent first step to finding the right people to go to in each office for questions.
RPL Express Start Resource:
Explore the offices and resources in the Workbook Companion that was provided as part of RPL Express Start Module 2: Navigating Clinical Research. Understand what they do, what they offer, and how your unit works with them.
LinkedIn Learning Videos:
NOTE: Duke no longer sponsors free LinkedIn Learning accounts. If you are a LinkedIn Learning user, your local library may provide free access if you have a library card (ex: Durham County Library).
- Interpersonal Communication (37 min)
Other Resources:
Resources
- Review the Issue and Change Management Tools from the RPL Project Management Repository
- RPN Session Recording – Holding a Space for Possibility: Flexible Thinking at Work and Home
- Duke Center for Healthcare Safety and Quality – Well-Being Tools
- Duke Personal Assistance Service
LinkedIn Learning Videos
NOTE: Duke no longer sponsors free LinkedIn Learning accounts. If you are a LinkedIn Learning user, your local library may provide free access if you have a library card (ex: Durham County Library).
- Change Management Tips for Leaders (16 min)
- How to Be an Adaptable Employee during Change > Motivate Yourself to Become More Adaptable (4 min)
- Adaptive Project Leadership > Adaptive Thinking in Practice (3.5 min)
- Project Management Foundations > How to Manage Project Change (2.5 min)
- Leadership Foundations > Leading Change Through Agility and Resilience (3.5 min)
LinkedIn Learning Videos:
NOTE: Duke no longer sponsors free LinkedIn Learning accounts. If you are a LinkedIn Learning user, your local library may provide free access if you have a library card (ex: Durham County Library).
- Problem Solving Techniques > Asking the Five Whys (4 min)
- Root Cause Analysis: Getting to the Root > Working With Others to Find Root Causes of Problems (3.5 min)
- Root Cause Analysis: Getting to the Root > Fishbone Diagram (3 min)
- Critical Thinking and Problem Solving (Peruse full course, lots of good lessons here)
DOCR RPN and RPN+ Sessions on Communication and Teamwork (Hint: use CTRL+F to search for “communication” and "teamwork" topics)
RPL Express Start Lesson Transcripts:
TeamMAPPS (Team Methods to Advance Processes and Performance in Science) is a free training program developed by the University of Texas Medical Branch (UTMB). TeamMAPPS is a behavioral skills training program specifically developed to increase the collaboration and effectiveness of scientific teams and scientific team members.
LinkedIn Learning Videos
NOTE: Duke no longer sponsors free LinkedIn Learning accounts. If you are a LinkedIn Learning user, your local library may provide free access if you have a library card (ex: Durham County Library).
Communication
- Project Management Foundations > Techniques for Communicating Effectively (4 min)
- Holding Your Team Accountable > Communicating Clear Expectations (3 min)
- Communicating with Emotional Intelligence > Communicate Confidently and With Tact (4min)
- Facilitation Skills for Managers and Leaders > The Five Elements of Emotional Intelligence (4 min)
- Improving Your Listening Skills > How to adopt the mindset of listening (3 min)
- Leading From the Middle > Communicate with Partners (3 min)
- Writing Emails People Want to Read > How to Get Them to Write You Back (2 min)
- Writing Emails People Want to Read > How to Write a Terrible Email (2.5 min)
- Writing Emails People Want to Read > How to be Clearly Understood (2 min)
- Organization Communication > Selecting a Communication Channel (4 min)
- Managing Globally > How to Communicate Across Cultures (3.5 min)
Teamwork
- Project Leadership > What Makes High Performing Teams? (3 min)
- Project Leadership > Applying Motivation Techniques to Your Team (4 min)
- Project Management Skills for Leaders > Build a Culture of Candor (2 min)
- Communication within Teams > Provide Feedback Within Teams (3 min)
- Communication within Teams > Manage Conflict Within Teams (5 min)
- Essentials of Team Collaboration > Best Practices to Enhance Team Collaboration (2.5 min)
- Collaborative Leadership > Collaborating Across Cultures (3.5 min)
- Leadership Foundations > Increasing Team Effectiveness (2.5 min)
- Facilitation Skills for Managers and Leaders > Engaging Conflict Productively (4 min)
- Leading Inclusive Teams > Prioritizing Inclusion on Your Team (3.5 min)
Competencies Specific to Research Program Leader Role
RPL Onboarding Engagement Activity Packets: Lead Project and Program Staff, Budgeting and Resource Management
LinkedIn Learning Videos Related to Operational Leadership
NOTE: Duke no longer sponsors free LinkedIn Learning accounts. If you are a LinkedIn Learning user, your local library may provide free access if you have a library card (ex: Durham County Library).
- Project Management Foundations > Manage Team Resources (3.27 min)
- Project Management Skills for Leaders > Delegate Tasks With Confidence (3 min)
- Holding Your Team Accountable > Tracking Team Performance (3 min)
- Holding Your Team Accountable > Accountability in Cross-Functional Teams (4 min)
- Performance Management: Setting Goals and Managing Performance (~20 minutes (hint – 10 minutes at 2x speed J))
- Manage Employee Performance Problems > Assess Employee Performance Problems (2 min)
- Manage Employee Performance Problems > The Coaching Conversation (4 min)
- Developing Team Members > Avoid Team Development Pitfalls (2.21 min)
- Project Management Foundations > How to Plan Procurement (3min)
- Project Management Foundations > Develop a Project Budget (3.5min)
- Project Management Foundations > Techniques for Communicating Effectively (4 min)
- Holding Your Team Accountable > Communicating Clear Expectations (3 min)
- Communicating with Emotional Intelligence > Communicate Confidently and With Tact (4min)
- Facilitation Skills for Managers and Leaders > The Five Elements of Emotional Intelligence (4 min)
- Improving Your Listening Skills > How to adopt the mindset of listening (3 min)
- Leading From the Middle > Communicate with Partners (3 min)
- Writing Emails People Want to Read > How to Get Them to Write You Back (2 min)
- Writing Emails People Want to Read > How to Write a Terrible Email (2.5 min)
- Writing Emails People Want to Read > How to be Clearly Understood (2 min)
- Organization Communication > Selecting a Communication Channel (4 min)
- Managing Globally > How to Communicate Across Cultures (3.5 min)
Helpful Resources for Project Management
- RPL Project Management Tools and Templates Repository: Bookmark the RPL Project Management Tools and Templates Repository in Duke Box for quick access to helpful templates related to the topics below. If you find resources that would be helpful to add here, send them to wer-jobs@duke.edu.
- Research Professionals Network Topic Recordings: Check the DOCR Research Professionals Network (RPN) site for helpful project management topics, such as "Task Management Practices and Tools," "Clinical Quality Management Program," "Team Science," and "Project Management for Clinical Research"
- Duke PMCOP: Duke Project Management Community of Practice (PMCOP) is a professional network at Duke that connects Project Managers across disciplines. They provide relevant training events and resources. Join the PMCOP to take advantage of their offerings.
- myRESEARCHpath: For navigating clinical research at Duke, please take advantage of myRESEARCHpath and myRESEARCHhome. You'll find much of the information you need, relevant training, and the right people to involve, at each stage of a clinical study.
- DCRI Research Navigator: The Duke Clinical Research Institute Research Navigator is the DCRI’s version of MyRESEARCHPath. While processes may not completely align for studies outside of the DCRI unit, the tool houses many great templates for different phases of project management.
RPL Engagement Activity Packets Related to Project Management:
- Project Initiation and Scope Management
- Project Planning
- Communication Planning and Stakeholder Management
- Task Scheduling and Management
RPL Express Start Lesson Transcripts and Resources Related to Project Management
- Intro to Project Management
- Project Management Lifecycle
- RPL Guide for Getting to Know Projects – Meeting with Leadership
- Protocol and Portfolio Review Considerations Checklist
- Creating a Project Plan
- Managing and Reporting Risk
- Quality Management
- PMCOP Project Management Software Applications InventoryPutting Together a Communication Plan
- How Project Managers Communicate Successfully
- Project Scheduling and Task Management
- Creating a Resource Plan
- Writing a Project Status Report
LinkedIn Learning Videos
NOTE: Duke no longer sponsors free LinkedIn Learning accounts. If you are a LinkedIn Learning user, your local library may provide free access if you have a library card (ex: Durham County Library).
Initiating Projects and Managing Scope
- Project Management Foundations > Initiate a Project (1.5 min)
- Why Projects Fail > Identify and Align with Organizational Goals and Objectives (3.5 min)
- Project Management Foundations: Small Projects > Define Project Scope (2.5 min)
- Project Management Foundations > Prepare a Project Scope Statement (2.21 min)
- Why Projects Fail and How to Improve Their Success > Avoid Scope Creep (3.5 min)
Project Planning
- Project Management Foundations > Project Planning Overview (1.5 min)
- Project Management Tips > What Does Project Management Software Do? (1 min)
- Project Management Foundations: Quality > Defining Project Quality (2.5 min)
Stakeholder Management
- Why Projects Fail and How to Improve Success > Stakeholders Have Needs and Expectations (4min)
- Why Projects Fail and How to Improve Success > How Communication Improves Project Success (4min)
- Project Management Tips > Build a Communication Plan (1.5min)
- Interpersonal Communication > How to Make Requests Effectively (3 min)
- Delegating Tasks > How to Communicate Effectively (2 min)
Task Scheduling and Management
- Project Management Simplified > Three Ways to Identify Project Tasks (3 min)
- Project Management Foundations > Build a Project Schedule (2 min)
- Why Projects Fail and How to Improve Their Success > Identify the Right Resources (5 min)
- Managing Resources Across Project Teams > Selecting People to Join Your Project Team (3.12 min)
- Project Management Foundations: Schedules > Procure Resources (2 min)
Milestone Tracking and Reporting
- Project Management Foundations: Communication > What to Include in a Project Report (3min)
- Business Writing Principles > Special Considerations for Reports (7 min)
- Project Management Skills for Leaders > Provide Status Update Guidance (3 min)
- Data Driven Project Management > Selecting Project Metrics That Matter (3.5 min)
- Data Visualization: Storyline > Data Storytelling is Essential (6 min)
RPL Onboarding Engagement Activity Packet: Intellectual Contribution
The Duke Medical Center Library and Archives offers training classes, consultations, and resources on scholarly communications and tools.
Linked In Learning Videos:
NOTE: Duke no longer sponsors free LinkedIn Learning accounts. If you are a LinkedIn Learning user, your local library may provide free access if you have a library card (ex: Durham County Library).
- Writing a Proposal > An Overview of Proposal Writing (5min)
- Grammar Girl’s Quick and Dirty Tips for Better Writing (40min – lots of good lessons here if helpful!)
- Academic Research Foundations: Quantitative > Purpose of a Literature Review (2.45 min)
- Academic Research Foundations: Quantitative > How to Develop a Literature Review (3 min)
- Academic Research Foundations: Quantitative > How to Present Your Data in Written Form (3 min)
- Designing Big Data Healthcare Studies > Tools for Reviewing the Scientific Literature (4min)
- Preparing for Successful Communication > Know Your Message (4 min)
- Deliver Standout Technical Presentations > Three Principles of Powerful Communication (2.5 min)
- Deliver Standout Technical Presentations > Why Bullet Points Don’t Work (3.5 min)
- Deliver Standout Technical Presentations > Brain Hacks for Slide Design (6 min)
- PowerPoint: Eight Easy Ways to Make Your Presentations Stand Out > This isn’t a Brochure (3.5min)
- PowerPoint: Eight Easy Ways to Make Your Presentations Stand Out > Three is The Magic Number (4.5min)
RCR Research Communication and Data Dissemination Online Module - 00134075
The RCR Research Communication and Data Dissemination Track contains 3 modules: 1) Authoring and Publication 2) Data and Resource Sharing 3) Wider Communication of Research -- Link to more info on DOSI Site.