RPN+ New Hire Cohorts and Foundational Topic Series

NOTICE: The monthly RPN+ New Hire Cohorts are temporarily moving to a reduced schedule as of April 2025. Please continue to register new hires and we will reach out and gauge continued interest once we have enough registrations to form a cohort. We will post an update in the DOCR newsletter when the cohorts resume a normal schedule. If you have questions or if you are seeking mentorship for yourself or someone else right away, please reach out to wer-jobs@duke.edu. We are still accepting mentor nominations for anyone interested in becoming a mentor.
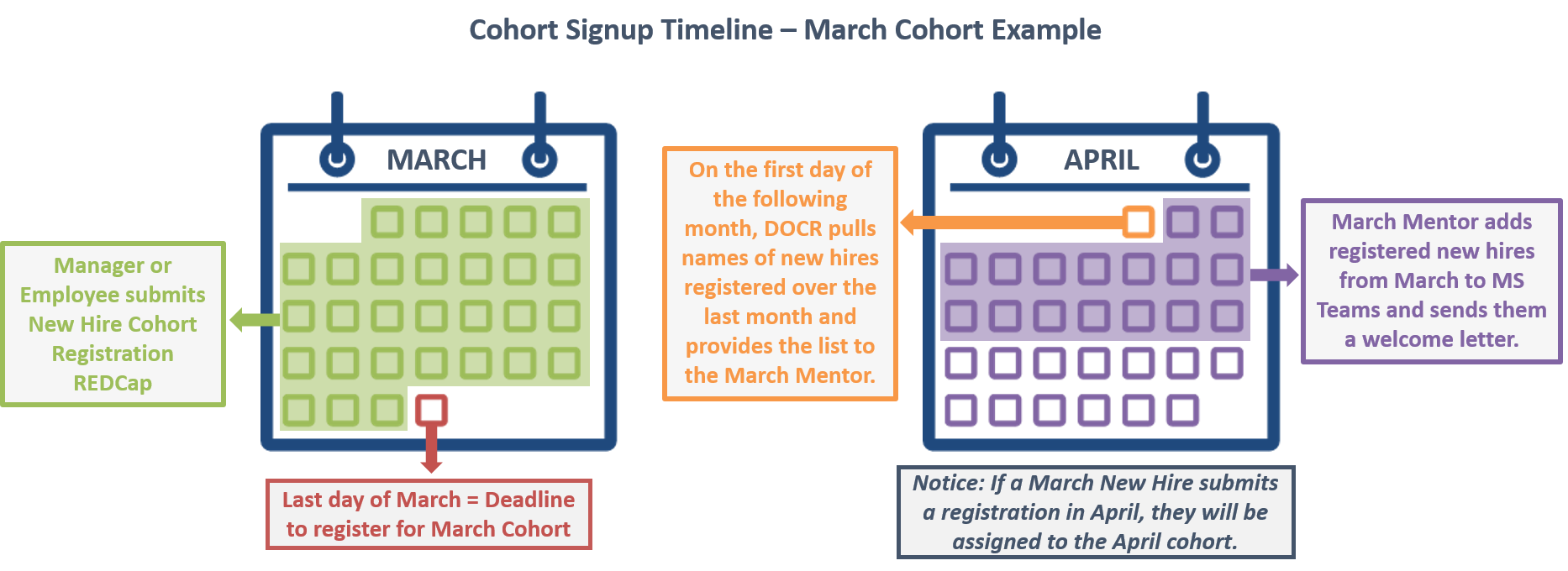
New hires in clinical research positions can elect to enter a New Hire Cohort with other staff hired in the same month. Each cohort will have an assigned mentor who will schedule and lead regular meetups, encourage community-building, and provide an open space for collaboration and communication. Cohorts will meet with their mentor as a group for 4-6 months.
Any new-hire or transfer in a clinical research role, from Clinical Research Specialist to Research Practice Manager, is welcome to join a cohort! Clinical research staff collaborate across jobs, so we think it is important to network and learn from people in a range of positions. (Estimated Time Commitment: 1-2 hrs per month)
Register yourself or an employee for the next cohort:
New Hire Cohort Registration Form
Become a Mentor for one of the New Hire Cohorts!
Mentors will manage a Microsoft Teams channel for their assigned cohort and schedule and lead two meetups each month for at least 30 minutes. These meetings may consist of a community-building activity, discussion about a topic of interest, and/or chatting about any challenges or questions. DOCR provides a toolkit of templates and topic ideas to take some burden of planning meetup topics off the mentor. The mentor will be expected to meet with their cohort each month for at least 4 months. Bonus: you can use this experience as evidence of leadership for Tier Advancement! (Estimated Time Commitment: ~3-4 hrs a month for at least 4 months)
Program Goals
-
Provide a networking and social element to new hire onboarding. Mentors will meet with their cohort of new hires bi-weekly. We encourage mentors to offer network-building opportunities at these meetings and get creative with how to make these meetings both helpful and social learning experiences.
-
Build collaboration and community across clinical research positions. We hope that by welcoming all clinical research positions into each cohort, we can build a community of clinical research staff who understand the roles around them and can collaborate effectively.
-
Provide a leadership opportunity for experienced clinical research staff. The mentor experience is intended to help experienced staff build or expand their leadership skills. We encourage mentors to make this opportunity their own and use this experience to grow their Tier Advancement portfolio if applicable.
RPN+ Foundational Series sessions are a collaboration between DOCR and CRU staff to host a monthly special Research Professionals Network session on a foundational clinical research topic. These sessions are proposed and facilitated by experienced clinical research staff. Everyone in the clinical research space is welcome to attend if a topic is interesting and relevant to them. New hires are especially encouraged to take advantage of these offerings.
A full list of upcoming topics is available on the RPN Webpage.
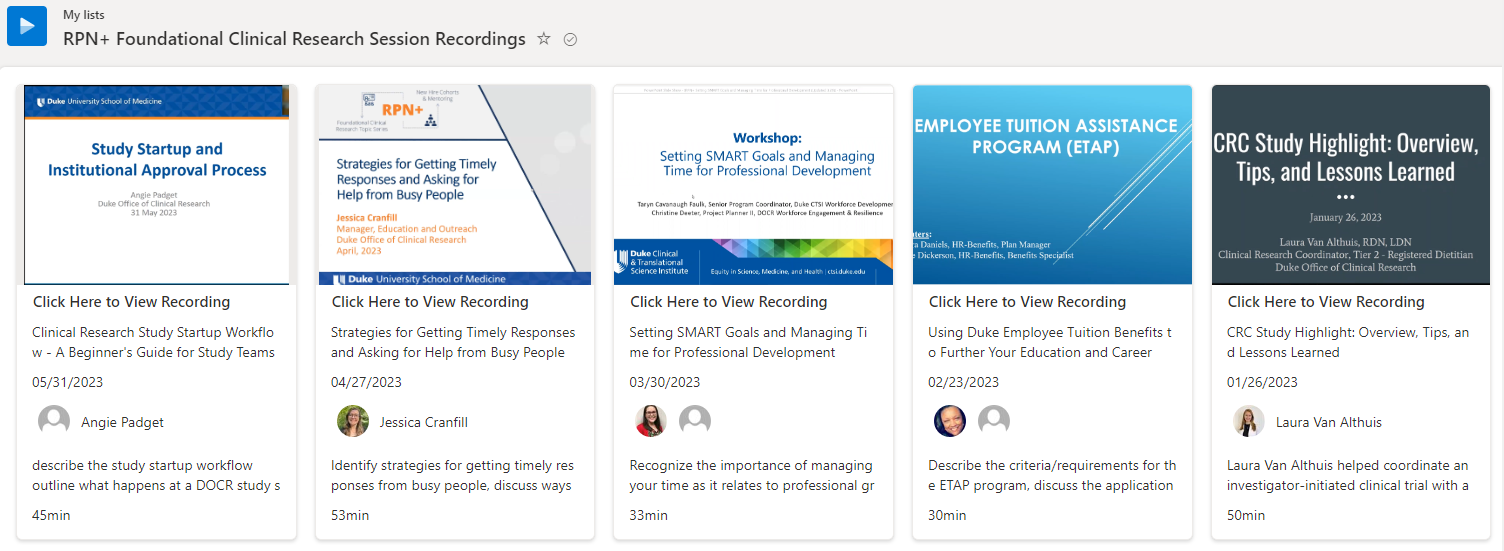
Check out our RPN+ Recording Library for a library of previous session recordings.
Facilitate a Session for the RPN+ Foundational Series!
These monthly sessions are proposed and facilitated by experienced clinical research staff. Topics might include things such as effective communication, collaborating with your PI, or SOP writing. Do you perform a certain function well or want to highlight your knowledge in an area that you think would be helpful to others? If so, submit a presentation proposal! (Estimated Time Commitment: 30 min to 1 hr facilitation, prep time may vary based on the individual and topic)
RPN+ Fundamental Topic Series Goals
- Create a community for new clinical research staff
- Offer helpful fundamental training topics for clinical research staff
- Provide a leadership opportunity for experienced clinical research staff
Express Start Online Modules
Express Start comprises a set of self-paced e-learning courses categorized by roles, designed to provide a comprehensive introduction to clinical research at Duke. The objective is to facilitate a swift "getting started" process for individuals entering the field of clinical research. These courses cover essential topics such as an overview of clinical research activities, relevant regulations, and role-specific workflows. Express Start is featured as the initial step in the Onboarding Learning Plan tool for each respective role.If you encounter any broken links in Express Start please let us know using this form.
Express Start Onboarding - CRS | 00164407 | Online
Express Start for CRS and CRS Sr. consists of three self-paced e-learning courses that will provide an overview of clinical research activities, support offices, and workflows at Duke. Click here for an outline of what is included in CRS Express Start.
Learning Objectives:
- Describe the roles and responsibilities of a Clinical Research Specialist
- Identify the clinical research offices and resources within Duke Health
- Identify the clinical research systems used at Duke and understand how they interact with one another
Express Start Onboarding Program-CRC | 00141671 | Online
Express Start for CRCs consists of four self-paced e-learning courses that will provide an overview of clinical research activities, regulations, and workflows at Duke. Click here for an outline of what is included in CRC Express Start.
Learning Objectives:
- Describe the roles and responsibilities of a Clinical Research Coordinator
- Identify the clinical research offices and resources within Duke Health
- Identify the clinical research systems used at Duke and understand how they interact with one another
- Discuss the studies that make up your research portfolio
- Describe the career progression within clinical research professions
Express Start Onboarding Program - CRNC | 00164563 | Online
Express Start for CRNCs consists of four self-paced e-learning courses that will provide an overview of clinical research activities, regulations, and workflows at Duke. Click here for an outline of what is included in CRNC Express Start.
Learning Objectives:
- Describe the research role and responsibilities of a Clinical Research Nurse Coordinator
- Identify the clinical research offices and resources within Duke Health
- Identify the clinical research systems used at Duke and understand how they interact with one another
- Discuss the studies that make up your research portfolio
- Describe the career progression within clinical research professions
- Define nursing responsibilities, competencies, and credentialing for the CRNC role
Express Start Onboarding Program-Regulatory Coordinator | 00145771 | Online
Express Start for Regulatory Coordinators consists of four self-paced e-learning courses that will provide an overview of clinical research activities, regulations, and workflows at Duke. Click here for an outline of what is included in Reg Coordinator Express Start.
Learning Objectives:
- Describe the roles and responsibilities of a Regulatory Coordinator
- Identify the clinical research offices and resources within Duke Health
- Identify the clinical research systems used at Duke and understand how they interact with one another; with a spotlight on iRIS
- Find and follow applicable standards, guidelines, regulations, policies, and procedures for performing clinical research at Duke
- Describe the career progression within clinical research professions
Express Start Onboarding Program-RPL | 00172350 | Online
Express Start for RPL consists of five self-paced e-learning modules that should be completed by individuals hired into the RPL and RPL Sr. positions at Duke. The WE-R Express Start Onboarding modules provide new hires in clinical research roles at Duke with an understanding of the “lay of the land” of clinical research at Duke. By completing these self-paced online modules, new RPLs will learn basic information that will begin to prepare them to manage clinical research projects and programs.
Click here for an outline of what is included in RPL Express Start.
Learning Objectives:
- Describe the roles and responsibilities of a RPL
- Identify the offices, programs, and resources that support clinical research at Duke
- Recognize the structure of your team and roles you can expect to interact with
- Identify the clinical research systems used at Duke and recognize how they interact with one another
- Recall best practices for becoming familiar with your clinical research portfolio of studies
- Describe the fundamentals of project management in a clinical research context
Express Start Onboarding - New Managers of Clinical Research Professionals | 00189215 | Online
New Manager Express Start comprises three self-paced eLearning modules tailored for individuals stepping into a manager role that will supervise Clinical Research Professionals in clinical research job codes at Duke. These modules are designed to complement the role-based Express Start Onboarding modules for Clinical Research Professionals (CRPs) and offer guidance for managing and supervising teams effectively. Through completing these modules, new managers will gain insights into their general responsibilities and acquire strategies for leading and retaining CRPs.
This training will be helpful for new CRPs hired into a manager role, CRPs transitioning into manager roles, and seasoned managers seeking a refresher.
Click here for an outline of what is included in New Manager Express Start.
Modules and Objectives Include:
- Your Role as a Manager of Clinical Research Professionals
- Recognize the importance of your role in connection with Duke values.
- Describe your role as a manager of clinical research professionals.
- Connect leadership competencies with strategies for leading your direct reports.
- Find resources and tools for getting started in your role.
- Overview of WE-R Initiatives and Tier Advancement for CPR Managers
- Describe clinical research hiring, onboarding, and tier advancement.
- Recognize manager responsibilities related to these WE-R initiatives.
- Identify how to partner with the WE-R team to make the most of available resources.
- Reflecting and Setting Goals for the Path Ahead
- Shift your focus from setting goals as an individual to setting goals as a manager.
- Recognize common manager goals and review tips to achieve them.
- Discuss the Five Practices of Exemplary Leadership in the context of clinical research.
- Reflect on who you want to be as a manager given what you have learned so far.
- Identify strategies to continue your learning journey.
Express Start Onboarding Program-RPM/ARPM | 00155742 | Online
Express Start for RPM/ARPM consists of five self-paced e-learning courses that will provide an overview of clinical research activities, regulations, and workflows at Duke. By completing these modules, new CRU leaders will learn basic information that will begin to prepare them for their role as a RPM or ARPM at Duke. Click here for an outline of what is included in RPM Express Start.
Learning Objectives:
- Describe the roles and responsibilities of a RPM or ARPM
- Identify the offices, programs, and resources that support clinical research at Duke
- Recognize the structure of your team and roles you can expwct to manage and interact with
- Identify the clinical research systems used at Duke and recognize how they interact with one another
- Recall regulations, guidelines, and policies for conducting clinical research at Duke
- Describe the RPM and ARPM responsibilities related to WE-R initiatives and Tier Advancement for employees
Express Start Onboarding - CRU Director | 00168054 | Online
Express Start for the CRU Director consists of one self-paced e-learning module that will acquaint new Clinical Research Unit (CRU) Directors (also referred to as CRU Medical Director) with the structure and expectations of their role within the CRU, the responsibilities of the other roles within the CRU, support available across Duke for clinical research, and other items that are important for the CRU Director to know. The course is intended for CRU Directors or others acting in a similar capacity for a CRU. Click here for an outline of what is included in CRU Director Express Start.
Lessons:
- Welcome to Your New Role (2 min)
- Clinical Research Units and Oversight Organizations (6 min)
- Your Role as a CRU Director (6 min)
- Clinical Research Personnel Roles Within Your CRU (12 min)
- Clinical Research Support Offices, Programs, and Resources at Duke (15 min)
- Intro to Workforce Engagement and Resilience (WE-R) Initiatives (8 min)
- Getting Started in Your Role (8 min)
Express Start for clinical research interns includes two self-paced online modules. In the first module, interns will learn about the purpose of a clinical research internship, the role of an intern, how to take ownership of the experience, and general professional expectations of interns. In the second module, interns will learn about taking a “Time Out” when they are interacting with information about the study and study participants and if they come in contact with sensitive data.
- Module 1: Getting Started with Your Clinical Research Internship
- Module 2: Clinical Research Intern “Time Out” Training: Pausing to Protect Study Participants + Their Privacy
Express Start for clinical research internship preceptors includes one self-pased online module. The module will provide clinical research internship preceptors with an understanding of what it means to host a clinical research intern, intern preceptor responsibilities, what a successful internship looks like, and how to determine what activities interns can participate in.
Learning Objectives and Descriptions:
|
Getting Started with Your Clinical Research Internship |
The goal of this training is to provide clinical research interns with helpful information as they get started with their internship. Interns will learn about the purpose of a clinical research internship, the role of the intern, how to take ownership of the experience, and general professional expectations of interns. This training will be required for clinical research interns onboarded through the Clinical Research Internship Portal (CRISP) Upon completion, interns will be able to:
|
|
Clinical Research Intern “Time Out” Training: Pausing to Protect Study Participants + Their Privacy |
The goal of this training is to help clinical research interns think critically when they are in certain situations during an internship. They will learn about taking a “Time Out” when they are interacting with information about the study and study participants and if they come in contact with sensitive data. This training will be required for clinical research interns onboarded through the Clinical Research Internship Portal (CRISP) Upon completion, interns will be able to:
|
|
Clinical Research Internship Preceptor Training |
The goal of this training is to provide clinical research internship preceptors with an understanding of what it means to host a clinical research intern, intern preceptor responsibilities, what a successful internship looks like, and how to determine what activities interns can participate in. This training will be required for individuals who will host clinical research interns onboarded through the Clinical Research Internship Portal (CRISP). Upon completion, you will be able to:
|
CRP Onboarding Learning Plan Templates
DOCR has developed Competency-Based Onboarding Learning Plan templates for the following Clinical Research Professional (CRP) positions at Duke. Templates are updated regularly as feedback is received and as new training becomes available. Prior to beginning onboarding for a new employee, managers should download the most recent version of the learning plan to ensure links and available training are current.
Clinical Research Specialist | Clinical Research Coordinator | Clinical Research Nurse Coordinator
Regulatory Coordinator | Research Program Leader
Access the Templates and a Manager Guide using the links below:
Onboarding/Training Checklists for Leadership Roles
DOCR has developed onboarding and training checklists for CRU leadership roles, including RPMs, ARPMs, CRU Directors and a PI Training Checklist that can be used to train new PIs or as a refresher for different topics. If you have feedback on these tools, please reach out to wer-jobs@duke.edu.
We review and update these as often as possible. If you encounter any broken links in these templates please let us know using this form.
Onboarding for new RPMs and ARPMs is available in checklist form. Individuals can request this checklist from DOCR by completing this form. The form is used to alert DOCR of your need for the most recent checklist, and to track onboarding plan use.
The checklist for RPMs and ARPMs includes:
- Suggested RPM and ARPM Training, including Express Start
- Suggested meetings to get acquainted with clinical research support offices at Duke
- Access needed for RPMs and some ARPMs - and contacts/resources for that access
- A list of specific tasks to complete as applicable to the CRU
When you receive the checklist using the form above, follow instructions for getting DOCR help with setting up necessary introduction meetings and system and reporting access for the new RPM or ARPM.
Onboarding for new CRU Directors is available in checklist form using the button below. CRU Directors can register use of the checklist and request meetings listed on the checklist using this form.
The checklist for CRU Directors includes:
- Suggested CRU Director Online Training, including Express Start and OnCore Modules
- Suggested introductory meetings and 1 on 1 training for CRU metrics and scorecards
- Access that may be needed for CRU Director - and contacts/resources for that access
- A list of specific tasks to complete as applicable to the CRU
The CRU Director, or person preparing their checklist, should follow instructions for submitting this form to receive DOCR help with setting up necessary introduction and 1 on 1 training meetings.
Updated April 9, 2025
This tool, developed by DOCR in collaboration with Medicine CRU, is an editable checklist that can be used for clinical research PIs. It includes a list of LMS training, policies, and other resources. These items are mapped to learning objectives that may be relevant or helpful to PIs who are getting started or who need a refresher on certain topics.
If you would like to suggest changes or additions to this checklist, reach out to DOCR.help@duke.edu.
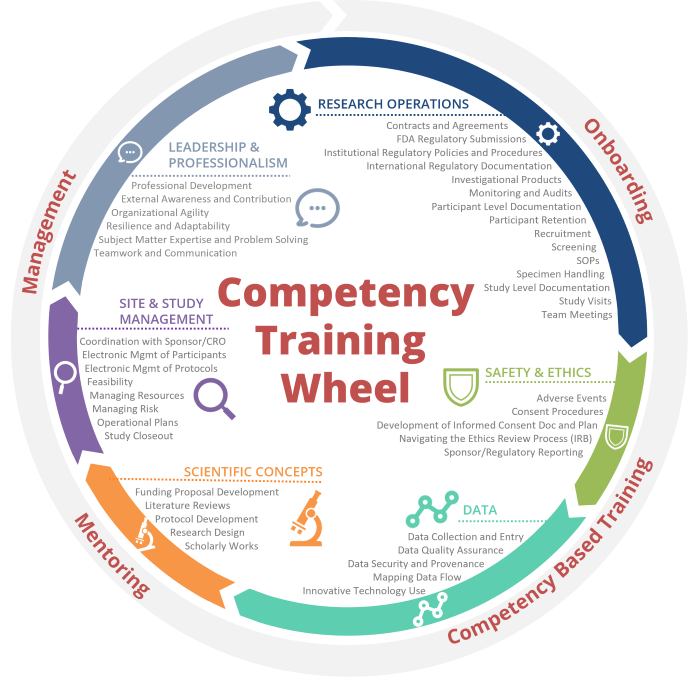
Training by Competency
The Training by Competency page includes training for competencies within each of the domains below. Click on a domain to find training that has been mapped to that topic on the Training by Competency page. A full listing of the competencies and associated assessments can be found on the WE-R Tier Advancement page.
Clinical Research Manager Training and Resources
The Workforce Engagement and Resilience (WE-R) team in DOCR collaborates with the Clinical Research Manager Training Committee and SOM HR partners to create periodic Manager Mini modules for the Clinical Research Update Newsletter and periodic manager training sessions. For suggestions on future topics, please contact wer-jobs@duke.edu.
New Manager Onboarding Learning Plan
Updated January 9, 2025
This onboarding learning plan template is intended for new managers of clinical research professionals. It can be used as a stand-alone learning plan for current employees transitioning into a supervisory role or added to the role-based Onboarding Learning Plan for new employees who are hired into a role that will manage other CRPs. Responsibilities for new managers may vary across CRUs; therefore, it is important to 1) review the plan and select training items relevant to the new manager's role, 2) provide additional project and unit-specific training as needed.
Included in this plan:
- Express Start for New Managers
- Resource Awareness
- Hiring and Onboarding New Staff
- Employee Training
- Research and Team Oversight
- Performance Management and Feedback
- Team Development and Advancement
- Employee Personal and Emotional Support
- Leadership and Professionalism
Express Start Onboarding - New Managers of Clinical Research Professionals | 00189215 | Online
New Manager Express Start comprises three self-paced eLearning modules tailored for individuals stepping into a manager role that will supervise Clinical Research Professionals in clinical research job codes at Duke. These modules are designed to complement the role-based Express Start Onboarding modules for Clinical Research Professionals (CRPs) and offer guidance for managing and supervising teams effectively. Through completing these modules, new managers will gain insights into their general responsibilities and acquire strategies for leading and retaining CRPs.
This training will be helpful for new CRPs hired into a manager role, CRPs transitioning into manager roles, and seasoned managers seeking a refresher.
Click here for an outline of what is included in New Manager Express Start.
Modules and Objectives Include:
- Your Role as a Manager of Clinical Research Professionals
- Recognize the importance of your role in connection with Duke values.
- Describe your role as a manager of clinical research professionals.
- Connect leadership competencies with strategies for leading your direct reports.
- Find resources and tools for getting started in your role.
- Overview of WE-R Initiatives and Tier Advancement for CPR Managers
- Describe clinical research hiring, onboarding, and tier advancement.
- Recognize manager responsibilities related to these WE-R initiatives.
- Identify how to partner with the WE-R team to make the most of available resources.
- Reflecting and Setting Goals for the Path Ahead
- Shift your focus from setting goals as an individual to setting goals as a manager.
- Recognize common manager goals and review tips to achieve them.
- Discuss the Five Practices of Exemplary Leadership in the context of clinical research.
- Reflect on who you want to be as a manager given what you have learned so far.
- Identify strategies to continue your learning journey.
These training snippets offer timely tips for managers and can be completed in under 15 minutes. They may include a variety of interactive elements such as templates, videos, and activities. All Manager-Mini Modules will be added to the LMS and archived here and in the DOCR Course List under "Manager Minis."
| Month | Module | Link |
|---|---|---|
|
June 2025 |
Managing Employee Stress with Care and Clarity This mini module is designed to give you simple, effective tools to help your team feel seen, supported, and grounded, even when you're managing your own stress. |
Register |
| April 2025 |
Overcoming Common Challenges as a Manager of Remote or Hybrid Teams |
Register |
| February 2025 |
From Ordinary Contributor to Impact Player |
Register |
| January 2025 |
Disseminating Information to Your Team |
Register |
|
October 2024 |
Crafting an Accountability Mindset |
Register |
| July 2024 | Strategies to Support Employee Engagement and Retention In this mini module, you’ll learn about types of employee turnover and review the top reasons employees disengage. Then, review strategies for keeping your team engaged including resources and tips suggested by your colleagues. Managers will receive downloadable tools to start off strong with new team members and a "Motivational Audit" to gauge current employee engagement. |
Register |
| May 2024 | Feedback and Feedforward In this mini-module, we will review types of feedback, a process you can use to provide feedback, how to deliver your message effectively, and tips to deal with certain responses. Managers will receive a downloadable 10-Step Process Checklist for providing feedback. |
Register |
| April 2024 |
Balancing Priorities and Managing Your Time |
Register |
| March 2024 |
Conducting Effective One-on-One Meetings |
Register |
DOCR collaborates with Duke University School of Medicine Human Resources and other teams to offer periodic virtual training sessions for managers of clinical research professionals. Recordings and slides are posted below.
February 2025 | Supporting Your Team - Duke Access and Accommodation Services
Description: This session is offered by Christina Kline, J.D., Executive Director for Duke Access and Accommodation Services. The session is a manager's guide to the Americans with Disabilities Act and Pregnant Workers Fainess Act and covers access and accommodation; specifically, manager responsibilities and ways that you can support your teams and connect them with Duke resources.
View Recording | View Slides
November 2024 | Timecards and Time Entry: Rules and Best Practices
Description: The third session in our series has been highly requested and will dive into time cards and time entry. This session is ideal for managers who want clear insights into policies, rules, and best practices for managing time-tracking processes.
View Recording | View Slides
August 2024 | Understanding Employee Resources and Leave Options for Staff
Description: This session covers available resources for your employees, including the offices at Duke that you should know about and how they can support your teams. Additionally, this session will provide a general overview of leave options for staff and leave administration (including Personal Leave, Family Medical Leave, and Parental Leave) and the option to seek a reasonable accommodation.
View Recording | View Slides
June 2024 | Performance Management and The Corrective Action Process
Description: The overall goal of the session is for managers to gain a foundational understanding of the progressive corrective action process, allowing them to work with their departmental HR to address concerns if they arise.
View Recording | View Slides
Competency-Based Continuing Education
The Engagement, Recruitment, and Retention (ER&R) Certificate Program is designed for clinical research study teams at Duke. It aims to enhance skills in engaging, recruiting, and retaining study participants while equipping staff with the tools and confidence to adopt practices that promote widespread participation.
Program Goals
- Develop engagement, recruitment, and retention specialists who can advocate for broad research participation and serve as resources for their clinical research units, departments, and divisions.
- Bolster the skills necessary for meaningful engagement, recruitment, and retention of clinical research participants.
- Assist study teams with strategies to engage participants and the community in research.
Want more information? Check out the program website.
Courses that are created and added to the Refresher Series will provide a refresher when attending a whole course on a broad topic isn’t necessary. Each series of micro-learning courses will provide a dive into a specific concept related to the overarching topic.
Study Documentation Refresher Series 1: Introduction to Clinical Research Documentation
This 12 to 15 minute module discusses the importance of study documentation and how to navigate different governing authorities as it relates to study documentation.
Study Documentation Refresher Series 2: Study Level Documentation
This 20 to 25 minute module discusses what study level documentation is, how to maintain it, and resources for creating and maintaining a compliant regulatory binder. You’ll also learn what documents are required for different types of studies and the importance of SOPs.
Study Documentation Refresher Series 3: Participant Level Documentation
This 15 to 20 minute module discusses what documents are “participant level” along with some specifics around informed consent, research data, and source data documentation. You’ll also learn how case report forms are used to report research data to the sponsor.
Study Documentation Refresher Series 4: How To’s and Best Practices
This 10 to 15 minute module discussed best practices for maintaining study documentation, proper practices for notation of changes, and when a note/memo to file might be required. You’ll also hear about when a protocol deviation would require both a Note/Memo to File and reporting to the IRB.
Study Documentation Refresher Series 5: Document Retention
This 10 to 15 minute module discusses how documents are retained and stored for open and closed studies as well as how regulations inform document retention requirements. You’ll also learn tips on planning for document storage and retention.
Onboarding Consultations for CRP Managers at Duke
If you are a manager of clinical research staff at Duke who is interested in using these tools and templates to onboard your new hires and don't know where to start, request a free consultation. We are happy to discuss your current onboarding process, review the DOCR onboarding tools with you, and help you determine how best to incorporate them into current onboarding practices in your Duke Clinical Research Unit.
External Access to Duke Onboarding Toolkit and Implementation Plan
Are you interested in accessing the Duke Clinical Research Professional Onboarding Toolkit developed by the Duke Office of Clinical Research? We are happy to share our Program Implementation Plan and Onboarding Toolkit repository of materials with other academic institutions for noncommercial and/or academic use to aid in implementing similar programming. Complete the form below to request access. You will also have the opportunity to request a free consultation with the Workforce Engagement and Resilience team if desired.
Publication: Cranfill JR, Deeter CE, Hannah D, Snyder DC and Freel SA (2023) Development and implementation of an on-demand competency-based onboarding program for clinical research professionals in academic medicine. Front. Med. 10:1249527. doi: 10.3389/fmed.2023.1249527
© Copyright 2025. Duke University. The copyrighted materials within the Clinical Research Onboarding Toolkit is licensed under CC BY-NC-SA 4.0. All other rights reserved. The toolkit was developed by the Duke Office of Clinical Research (DOCR) with support from the National Center For Advancing Translational Sciences of the National Institutes of Health under Award Number UL1TR002553 and is intended for noncommercial and/or academic use only. All other uses, including for-profit licensing requests, should contact wer-jobs@duke.edu or otcquestions@duke.edu and reference "OTC File 8242" for further licensing information.