Facility Highlights
-
Advanced Infrastructure: DEPRU features a 30-bed research facility equipped with clinical trial confinement spaces and six exam rooms. We also offer outpatient facilities for follow-up care and assessments, fully integrated with Duke Health's specialized medical services and equipment.
-
Comprehensive Participant Monitoring: Our facility ensures participant safety with 24/7 access to emergency medical personnel, wireless ECG telemetry for continuous cardiac monitoring, and capabilities for both invasive and non-invasive hemodynamic monitoring.
-
Specialized Research Services: We provide study-specific nutrition planning, access to Duke’s investigational drug services, imaging, and genomic analysis, along with expert guidance in clinical trial design and implementation.
Operational Expertise
-
Project Management and Study Coordination: Our specialized team offers tailored solutions, including project management, study coordination, and data management, ensuring efficient delivery with a single point of contact for streamlined collaboration.
-
Recruitment Core: DEPRU's dedicated recruitment specialists utilize advanced technology and strategies to identify and engage participants, managing a large registry of healthy volunteers and employing targeted approaches for disease-specific populations.
-
Laboratory Services: Our experienced laboratory technicians provide reliable support, including expert specimen collection and processing, immediate shipping and secure storage capabilities, and point-of-care testing.
Operational Capabilities
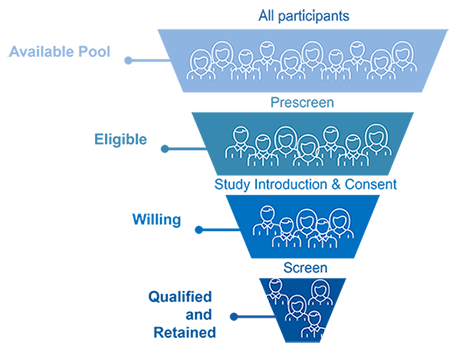
Diverse Participant Pool:
- Over 7,500 registry participants, including both healthy volunteers and disease populations.
- High retention rates and return participation.
Targeted Recruitment Strategies:
- EHR query systems to identify patient populations.
- Social media, advertisements, and patient referrals to maximize outreach.
- Automated messaging and follow-ups for participant engagement.
- Accredited facilities offering specimen handling, secure storage, and rapid testing.
- Point-of-Care (POC) testing for timely and accurate results.
- Robust systems for secure data collection and reporting.
- Expertise in protocol development and regulatory compliance.
- Flexible service locations and seamless transitions to later-phase studies.
- Broad-ranging testing and procedural capabilities tailored to your needs.
Partner with us to unlock innovative solutions, unmatched expertise, and seamless support for your clinical research journey.
For any business development inquires, including requesting a proposal or feasibility review of an opportunity, please contact:
Jessica Ashworth, Research Program Leader jessica.ashworth@duke.edu
Wan Lan Liang, Research Practice Manager wan.liang@duke.edu
You might consider having a Mutual Confidentiality Agreement in place.