 Our submission requirements have been carefully optimized for experimental success and efficiency. Researchers may only submit samples once an order is placed.
Our submission requirements have been carefully optimized for experimental success and efficiency. Researchers may only submit samples once an order is placed.
To minimize workflow disruptions for everyone’s projects, we will return submitted samples that do not adhere to the following requirements:
- Suspend samples in dH2O, 10mM Tris, or Qiagen EB. Samples in high salt buffers will be cleaned for an extra charge as salt interferes with library preparation.
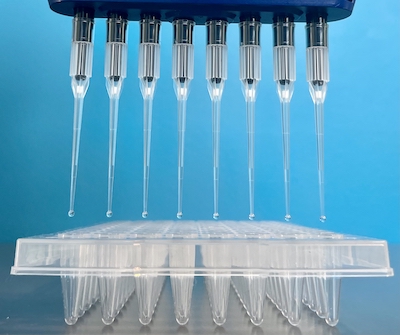
- Load samples on a clear semi-skirted polypropylene PCR plate going down the columns (Sample 1 = A1, sample 2 = B1, sample 3 = C1, etc.) as depicted above.
We do not accept full-skirted, deep well, round-bottom, or flat-bottom plates. - Label plates with the order number and sample number range (e.g 4596_S01-S96) and seal firmly with a foil plate seal capable of withstanding -80o C temperatures. If you have more than one plate, number the plates (e.g. 4596_S01-S96_Plate1, 4596_S97-S192_plate2).
- If you submit one sample, you may submit in a 1.5mL Eppendorf tube with your order number written on the top of the tube.
In addition to the plating requirements above:
| Library Preparation Type | Library Prep Kit Used | Volume Range (µl) | Input Range (ng) |
|---|---|---|---|
| DNA-Seq with Shearing | Watchmaker DNA Library Prep with Fragmentation | 30 | 300 |
| DNA-seq (full volume reaction) | llumina DNA Tagmentation | 30 | 300 |
| DNA-Seq (1/4 volume reaction) | llumina DNA Tagmentation -384 samples | 15 | 300 |
| Whole Exome Library | TWIST Human Whole Exome Panel | 30 | 300 |
| Methyl-Seq | NEBNext Enzymatic Methyl-Seq | 30 | 300 |
| ChIP-Seq | Watchmaker DNA Library Prep | 30 | 300 |
| Custom panels and targeted sequencing | contact sequencing@duke.edu |
In addition to the plating requirements above:
| Library Preparation Type | Library Prep Kit Used | Volume Range (µl) | Input Range (ng) | RIN |
|---|---|---|---|---|
| polyA tail enrichment - stranded mRNA-seq | Watchmaker mRNA Library Prep Kit | 30 | 300 | ≥ 7 |
| Ribosome depletion stranded RNA-seq | Illumina Tru-Seq Stranded Total RNA-seq kit W/ Ribo-Zero | 30 | 300 | DV200>50% |
| Ribosome and Globin depletion stranded RNA-seq | Tecan Universal Plus mRNA-seq | 30 | 400 | ≥ 7 |
| Low RNA input RNA-Seq - Not stranded | Takara SMART-seq Ultra-low input mRNA-seq kit followed by Watchmaker RNA Library Prep | 15 | 1 to 25 | ≥ 7 |
| small and microRNA-Seq | QiaSeq miRNA Library Prep | 30 | 300 | ≥ 7 |
| FFPE and degraded RNA | contact sequencing@duke.edu |
We accept prepared libraries for sequencing; however, we do not guarantee the sequencing results for any customer prepared libraries.
Please provide as much information as possible (loading concentration, % PhiX, custom read lengths, protocol, pooling parameters, etc.) to increase the success of your sequencing run. We will load customer-prepared libraries according to the directions provided. If a run fails due to missing or erroneous directions (incorrect library or loading concentrations, mislabeled barcodes, incorrect sequencing protocol, etc.), it is the client's responsibility to pay for the failed sequencing run and any subsequence sequencing runs. If you have any questions before submitting your custom sequencing or client-prepared libraries, please contact us.
If you are submitting custom primers, you must provide a minimum of 10ul at 100uM. We do not provide custom primers.
In addition to the plating requirements above:
| Sequencer | Flow Cell | Min Concentration (nM) | Min Volume (ul) |
|---|---|---|---|
| MiSeq | -- | 10 | 20 |
| NextSeq | -- | 10 | 20 |
| NovaSeq X Plus | 1.5B | 20 | 50 |
| 1.5 Single Lane*** | 10 | 40 | |
| NovaSeq X Plus | 10B | 20 | 120 |
| 10B Single Lane*** | 10 | 40 | |
| NovaSeq X Plus | 25B | 20 | 200 |
| 25B Single Lane*** | 10 | 40 |
**Any clients requesting custom sequencing runs (e.g. custom primers, custom sequencing protocols for 10x, etc.) must purchase a full flow cell.
***Custom sequencing protocols can be run on NovaSeq X Plus lanes.
Blood Samples for RNA extractions (2.5ml) should be collected in PAXgene Blood RNA Tubes. Immediately after blood collection, gently invert the PAXgene Blood RNA Tubes 8–10 times. Store the PAXgene Blood RNA Tubes upright at room temperature (18−25 ̊C) for 2 - 72 hours before transferring to −20 ̊C. If tubes are to be kept at temperatures below −20 ̊C, freeze them first at −20 ̊C for 24 hours, then transfer them to −80 ̊C. Tubes should be delivered to us frozen. Note that the frozen PAXgene Blood RNA Tubes are very fragile and break easily. Tubes must be clearly labeled with your DUGSIM order number and sample number (“4596_S001”). For human blood samples, please make sure to provide us with your IRB number.
Cells for RNA extractions should be pelleted and snap frozen in Eppendorf or Sarstedt 2.0 mL tubes with liquid removed. Cell pellets should have between 1-3 million cells.