About Us
Duke Early Phase Research Unit (DEPRU), a service center, is a Duke School of Medicine Clinical Research Unit (CRU) positioned within the Duke Office of Clinical Research. DEPRU supports the design, development, implementation, and conduct of Phase I-II clinical trials, including first-in-human and proof-of-concept studies in healthy, special, and disease-specific populations.
The DEPRU CRU’s alignment with Research at Pickett Road also allows for seamless management of a diverse range of studies, including validation device studies and later phase research. We provide services to the entire Duke health system. Researchers from every department can utilize our resources to get their human research off the ground.
Mission Statement
Duke Early Phase Clinical Research conducts high quality and innovative early phase research to accelerate the availability of therapies, diagnostics, and medical devices to improve the health of every human being.
We specialize in delivering diverse and highly-specialized early phase clinical research solutions.
- Collaborative Partnerships: We work with pharmaceutical, biotech, and tech companies, as well as government agencies, foundations, and academic medical centers to fast-track the development of therapies, devices, and diagnostics.
- Tailored Study Designs: Our team of clinical trial experts creates customized study designs and implementation plans, leveraging deep expertise across various therapeutic areas.
- Reliable Data & Insights: Using industry-standard methods, we ensure efficient data collection, and interpretation to deliver dependable results you can trust.
- Phase 1: First-in-Human Trials
- Healthy Volunteers
- Escalating Dose Studies (SAD/MAD)
- Bioavailability/Bioequivalence
- Drug-Drug Interaction and Food Effect Studies
- Phase 0 Studies
- Hybrid and Special Populations
- Invasive Procedures and Monitoring
- Device and Technology Validation
- Imaging, Endotoxin, Sleep Studies, and More
Our integrated expertise ensures efficient execution, robust data collection, and high-quality outcomes for even the most complex early-phase trials.
Experience by the Numbers (Average Per Year)
With an impressive track record, we deliver unmatched expertise and efficiency in clinical research:
- 33 active studies spanning 21 diverse therapeutic areas annually.
- 7,500+ registry volunteers, with 53% being healthy participants.
- 5 audits per year (71 total since inception).
- 1,800 outpatient visits on average annually.
- 300+ confinement visits conducted annually.
- 7,000+ biospecimens processed each year.
Leadership
Contact Us
In addition to our state-of-the-art research unit, we collaborate with preferred vendors or your chosen partners to ensure the accurate collection, analysis, and interpretation of data using industry-standard methods.
Partner with us to leverage this depth of experience and robust infrastructure for your clinical research success.
For any business development inquires, including requesting a proposal or feasibility review of an opportunity, please contact:
DEPRUCRU-BD@duke.edu
Our Services
Plan for Success: The Path to Approval

Clinical trial success depends on overcoming common pitfalls. We offer the expertise and tools to deliver results efficiently and effectively.
Common Reasons for Clinical Trial Failure
- Site selection
- Study design
- Poor execution
- Inadequate recruitment
What We Offer
- Project Management Expertise
- Cost-effective use of time and budget resources without compromising integrity or compliance
- Collaboration to identify opportunities for cost savings
- Enrollment Expertise
- Proven strategies to ensure timely participant recruitment
- Meticulous Execution
- Establishes a firm foundation for subsequent trial phases
- Strategic Design
- Expert input on study protocols and design to optimize trial success
- Comprehensive Support
- Flexible service locations and seamless transitions to later-phase studies
- Broad-ranging testing and procedural capabilities tailored to your needs
Efficiency from Start to Finish
Our expertise in early-phase trial design and execution translates to efficiencies that benefit late-phase studies. By addressing challenges early, we help increase success rates for bringing promising products to approval.
Partner with us to achieve your clinical research goals with precision and confidence.
Our Resources
- Seamless integration for staffing with the DOCR Core Service Center for Clinical Research Specialists, Clinical Research Coordinators, and Regulatory Coordinators
- Clinical Research Professionals are onboarding and trained in alignment with the Workforce Engagement and Resilience Program
- Staffing needs are adjusted in response to protocol demands and timelines
- Accredited facilities offering specimen handling, secure storage, and rapid testing
- Point-of-Care (POC) testing for timely and accurate results
- Access to on-campus CLIA/CAP accredited clinical laboratory system
- Nursing staff capable of performing electrocardiograms (ECGs), phlebotomy, IV and port access, vital signs assessment, safety monitoring and administration of investigational products
- Advanced practice providers (APPs) available for physical exams, safety assessments, and clinical oversight
- Planning, start-up, and implementation oversight by experienced project leaders
- Coordination with study investigators, sponsor, and study teams to manage deliverables and maintain timelines
- Agility in study conduct through management of resource capabilities as part of the Duke School of Medicine and Duke Health System
DEPRU Facility

40 Duke Medicine Circle
Clinic 3N, Red Zone
Durham, NC 27710
We are located in the Duke Clinic building, which is connected to the Duke Medicine Pavilion and the Duke Cancer Center
Advanced Infrastructure
DEPRU features a 30-bed research facility equipped with clinical trial confinement spaces and six exam rooms. We also offer outpatient facilities for follow-up care and assessments, fully integrated with Duke Health's specialized medical services and equipment. The unit is located within the Duke South Clinic.
The DEPRU Unit Features

Security: The DEPRU Unit has immediate, around-the-clock access to an on-site, full-scale emergency response team and medical provider coverage. The DEPRU Unit also provides extensive security measures for research staff and participants, including alarms, controlled access locks, locked elevators, and Duke Security personnel on-site 24 hours a day.
Waiting Areas: The secure DEPRU unit has a dedicated volunteer waiting, check-in, and discharge area. All DEPRU unit visitors are required to sign in, are issued a wristband or name badge, and are then escorted to their meeting location. Approved secure badge access is provided to Duke personnel.
Examination and Overnight Rooms: The DEPRU unit has well-equipped examination rooms for screening, outpatient visits, or procedures. Adjustable examination tables, electric beds, and infusion chairs are available for use. The overnight stay area has up to 30 participant beds, and each room has access to highspeed internet and Apple TV. Access to the overnight stay rooms is restricted to those with appropriate credentials.
Equipment: The DEPRU unit has both Universal Cide Cart and AED for pediatric and adults, and utilizes the Omnicell automated dispensing cabinet and locked medication refrigerators. The Mortara Telemetry Central System with 16 telemetry channels and Mortara 12-lead ECG machines for continuous monitoring is available in the overnight stay area. The unit has Welch Allyn Vital Sign machines, Welch Allyn Connex Spot Monitors, and BD Alaris PCU infusion and syringe pumps.
Common areas: If protocol permits, participants can also access our multi-purpose room, a reading room, laundry facility, an outdoor patio, and a large comfortable lounge equipped with games, puzzles, and a high-definition television.
Investigational Pharmacy: The investigational Pharmacy has a secure on-site location for preparation and dispensing of study product, if needed by the protocol.
Duke Hospital Services: The DUH First Response Team (Team 115) is available for medical emergencies, and a hospital-based IV team with ultrasound-guided intravenous access capabilities is on hand as needed.
Processing Laboratory: The laboratory has two central wet laboratory work areas. All samples are assigned a unique specimen ID at the point of collection; upon receipt by the laboratory, samples are logged in and checked for accurate labeling and assessed for quality prior to processing.
Each laboratory space houses both a -20°C non-cycling freezer and a -80°C freezer. All freezers are on emergency power backup circuitry. Additional equipment includes: refrigerated centrifuges, one refrigerator (4.0°C), one refrigerator/freezer (4.0°C/-20.0°C), Ohaus Harvard Trip balances, laboratory eyewashes, laboratory safety showers, a biological safety cabinet, a chemical fume hood, ice machines, and micropipettes.
The processing laboratory is staffed by medical technologists experienced in clinical research protocols and sample handling. In addition to biospecimen processing & shipping, the lab team expertly handles all facets of specimen management, including a robust chain of custody. Samples are promptly processed, split, and frozen as required. Laboratory equipment is monitored and maintained. Capability for short-term storage (up to 90 days) or immediate shipment to central laboratories is available.
DUHS Clinical Labs
DUHS Clinical Laboratories are accredited by the College of American Pathologists. Support for protocol-required testing depends on the specific requirements of the research. The DUHS Clinical Laboratories organization is configured to meet the needs of both patients and research subjects, including the range of testing and turnaround time required for specific protocols. Research specimens are handled in similar fashion to incoming clinical samples. For tests not included in the laboratory portfolio, DUHS Clinical Laboratory can use an extensive network of referral labs.
Recruitment Excellence
Accelerated Enrollment via Targeted Recruitment
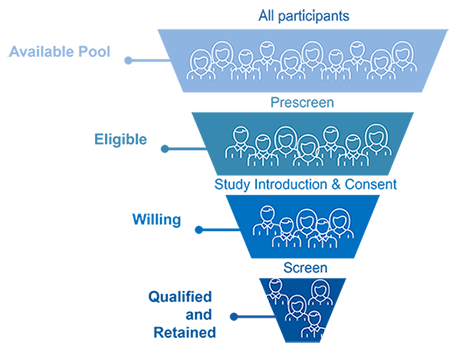
Duke Health Research Volunteer Registry
- Over 7,600+ consented volunteers and growing
- Includes:
- 4,076 registered healthy volunteers
- 3,588 volunteers with medical conditions
High Retention Rates and Return Participation
- Automated messaging and follow ups for participant engagement
-

- Identifies patient populations using ICD codes
- Facilitates automated patient messaging
Other Tools
-

- Patient referrals
- Phone outreach conducted by expert recruiters
- Identifies eligible subjects on arrival to the health system
- Alerts study personnel for streamlined enrollment
Volunteer for Research
Clinical Trial Participation
Clinical trials can lead to the development of new drugs, new surgical procedures and devices, that serve to advance healthcare and improve patient outcomes. They can also help doctors learn and advance their research. Many of these medical advances occurred because people volunteered to participate in clinical trials and other types of clinical research. People who participate in clinical trials want to help others, contribute to moving science forward, and also potentially receive investigational products.
The Duke Early Phase Research Unit’s top priority is to protect the health and wellbeing of participants.
Our purpose is to advance health care through research.
Early Phase Clinical Research
The Duke Early Phase Research Unit conducts Phase I clinical trials to assess the safety of investigational new drugs and devices (products that are not yet approved for sale in the U.S.) with the end goal of improving patient outcomes by contributing to the development of potential new treatment options.
Early phase research often involves administering an investigational product on healthy, adult volunteers before it is administered in patients.
We are seeking healthy and patient populations to register and participate in our studies.
For clinical research opportunities:
Volunteer Registry
We welcome anyone to join the Duke Health Research Volunteer Registry by clicking the link here.
New Clinical Trials Website and Directory
Duke University School of Medicine, in partnership with the Duke Clinical and Translational Science Institute (CTSI) and the Duke Office of Clinical Research (DOCR), has launched a new community-facing website about clinical research here.
Partner With Us

The DEPRU CRU is a comprehensive early phase research group with the experience, resources, and infrastructure needed to efficiently initiate and complete clinical research protocols.
The DEPRU CRU is one of a kind as a site partner option in that it has the speed capabilities and experience needed to successfully complete clinical protocols timely with our quality commitment.
We offer a number of advantages as a study execution partner that we have listed below for your consideration.
- Rapid start-up: Our efficient initiation process offers a competitive advantage in the race to complete clinical trials on time and within budget.
- Recruitment capabilities: Through our volunteer registry and patient database, we have access to a significant number research volunteers pre-identified as interested in participation in our research studies.
- Safety: Our hospital-based unit provides immediate, round-the-clock access to an on-site, full-scale, emergency response team and medical coverage.
- Thought leadership: As part of the Duke University School of Medicine, we benefit from the expertise of faculty and research professionals across the Institution.
- Cutting-edge technologies: We have on-site access to a full array of specialized core laboratories, including “omics,” advanced imaging, cell therapy, biobanking, and immune monitoring.
- Investigational Drug Service: We have on-site access to a dedicated research pharmacy.
- Experience: We have more than 25 years of early-phase clinical trial experience and have successfully conducted more than 200 early-phase studies, including Phase I trials.
- Operational excellence: Our staff members are experts in operational efficiency and regulatory compliance.
Partner with us to leverage this depth of experience and robust infrastructure for your clinical research success.
For any business development inquiries, including requesting a proposal or feasibility review of an opportunity, please contact: DEPRUCRU-BD@duke.edu


