Years in the Making: Duke Human Vaccine Institute Researchers Attack COVID-19
When the novel coronavirus began to spread across China, researchers at the Duke Human Vaccine Institute (DHVI) sprang into action and they haven’t slowed down since. They are collaborating with each other and with other institutions to unlock the secrets of the virus that causes COVID-19 and to develop tests, vaccines, and treatments.

“We’ve done more work faster than we have ever done before, making real progress on antibodies, tests, and a vaccine,” says Barton Haynes, MD, the Frederic M. Hanes Professor of Medicine and the director of DHVI.
DHVI researchers are attacking the new virus by applying knowledge, experience, and technology gained from years of working with HIV, influenza, and other viruses.
“All the work we’ve done has allowed us to transition on a dime,” Haynes says. “All the vaccine constructs we’ve developed were immediately repurposed for COVID-19.”
And vaccine designs are only the tip of the iceberg. DHVI faculty are also developing and running assays and tests, accessioning and distributing blood and tissue samples for studies, and isolating antibodies from infected individuals. Using approaches from a number of different disciplines, they are putting together a holistic picture of how the immune system and the novel coronavirus interact.
Two long-running programs at DHVI set the stage for the swift response. One is the HIV vaccine research program funded by the National Institute for Allergy and Infectious Disease (NIAID). A series of three grants from NIAID have provided continuous funding since 2005 of the Duke Center for HIV/AIDS Vaccine Immunology (CHAVI), Duke Center for HIV/AIDS Vaccine Immunology and Immunogen Design, and Duke Consortia for HIV/AIDS Vaccine Development (CHAVD). The other is a pandemic preparedness program funded since 2017 by the Department of Defense’s Advanced Research Projects Agency (DARPA).
“All the work we’ve done has allowed us to transition on a dime,” Haynes says. “All the vaccine constructs we’ve developed were immediately repurposed for COVID-19.”
The DARPA Pandemic Preparedness Platform (P3) seeks to develop strategies, technologies, supply chains, and expertise to make it possible to rapidly produce antibody-based treatments for any novel pathogen. These treatments, which can also be used for temporary prevention, are sometimes called passive vaccines or medical countermeasures.
Greg Sempowski, PhD, professor of medicine and pathology and leader of the P3 program, says, “I’m incredibly proud of how well our staff and scientists have stepped up. They have worked very long hours to quickly bring on all the systems needed to support this type of research. It’s not easy. Having really high-quality people who are committed is an enormous asset.”
DHVI’s work on COVID-19 is being supported with emergency funding from the National Institutes of Health (NIH), supplements to existing grants, and $17 million allocated to Duke by the North Carolina legislature.
Antibodies as a Treatment and Preventative
Antibodies play a crucial role in the development of vaccines, treatments, and even some kinds of COVID-19 tests, so the first order of business was discovering antibodies capable of neutralizing the new virus, SARS-CoV-2.
“Once we have the antibodies in hand, there are lots of different things we can do with them,” says Michael “Tony” Moody, MD, associate professor of pediatrics. “The key thing is getting the antibodies in hand.” Moody’s lab was one of several that collaborated to do just that.
DHVI researchers isolated more than 2,500 antibodies from individuals infected with COVID-19 in only ten weeks—a remarkable feat.

Kevin Saunders, PhD, associate professor of surgery and director of research at DHVI, says, “The antibody isolation technique that we use was developed under our HIV research programs over the last 15 years. We’ve really learned how to do that quickly and in depth. That’s why we could get to 2,500 antibodies in a matter of weeks.”
Of those antibodies, DHVI scientists have identified some with potent and complementary neutralizing powers against SARS-CoV-2. Together or individually, these antibodies could be a powerful treatment for people in the early stages of infection. They could also be used as a temporary preventative for people at high risk of exposure, such as healthcare workers.
One of these antibodies will be tested as a preventative in a Phase I trial in early 2021. Rather than manufacturing the antibodies, which is very time consuming, DHVI will manufacture mRNA molecules, the genetic blueprints that tell the body how to make the antibodies.
This effort is being supported by an additional $7.6 million grant from DARPA. Emmanuel “Chip” Walter, MD, professor of pediatrics, who directs the DHVI Clinical Trials Unit, will be running the trial. The manufacturing will happen onsite in DHVI’s current Good Manufacturing Practice (cGMP) facility, directed by Matthew Johnson, PhD.
These potent antibodies are also being used to create a test for COVID-19. Because they bind so well to the virus, the antibodies will attract SARS-CoV-2 like a magnet. This type of test could be faster and less expensive—and therefore more widely available—than polymerase chain reaction (PCR) tests.
Active Vaccines
A long-lasting vaccine, sometimes called an active vaccine to distinguish it from antibody treatments, is also a priority at DHVI.
An active vaccine spurs the body to create not only effective antibodies, but also “memory” cells that can churn out more of those antibodies in the future if needed.
Dozens of vaccine candidates are already being manufactured and tested around the world. This first wave of vaccines will doubtless slow down the spread of COVID-19, but it’s possible, even expected, that they will provide less than full protection.
DHVI is working on vaccines that will plug some of the holes. “We’re thinking about a second wave of vaccine with enhanced immunogenicity,” Saunders says. DHVI vaccines may be able to provide a boost to some of the front runners if they turn out to have a low potency or not to be effective in a particular population, such as older adults.
A multidisciplinary understanding of SARS-CoV-2 antibodies is crucial to this effort. “We really go deep,” Saunders says. “We’ve looked at a more global picture of antibody response.” That global picture is the necessary foundation for designing a highly effective vaccine.
“The power of the DHVI is that we have people who think about the problem in a different way, but we all come together and use our skill sets to make the biggest impact on the same problem,” Saunders says.
A Multidisciplinary Picture
Kevin Wiehe, PhD, associate professor of medicine, studies the genetic sequences of antibodies using computational methods. He looks at how the antibody sequences from people with COVID-19 evolve as their infections progress. “We normally do very deep sequencing so we can get hundreds of thousands of antibody sequences from an individual at any time point,” he says. “We can see the initial antibody response, which is potentially different than the [mature] antibody that occurs later.”
Other DHVI scientists are looking at the other side of the equation—the virus. SARS-CoV-2 is covered with spike proteins that allow the virus to infect cells. These spikes are where antibodies attach.
Rory Henderson, PhD, assistant professor of medicine, uses computer simulations to identify mutations in the spike protein that alter its shape, or conformation. In an actual infection, spike proteins change conformation frequently. But in a vaccine, some of these conformations will do a better job than others at spurring the immune system to produce effective antibodies. “It’s been remarkable how quickly we were able to go from not knowing anything about the coronavirus to having these designs,” Henderson says. “If one of the shapes is preferred, we already have that particle ready for a vaccine.”
This speed was made possible by previous HIV work. Henderson says, “We repurposed all of the techniques and tools we used for HIV and applied those to the coronavirus spike.”

Priyamvada Acharya, PhD, associate professor of surgery, puts it this way: “We have been studying a very difficult virus for a long time—HIV 1. So we have gained some superpowers.”
Acharya examines the engineered spike proteins at the atomic level in the Titan Krios cryo-electron microscope to make sure their shape is what was expected. Then the spikes can become ingredients in vaccines, either as mRNA or manufactured proteins. Indeed, some are already being evaluated in animal studies. Acharya uses the cyro-EM to take a look at samples from the studies to see if good antibodies are being produced and how they interact with the spike. So far, the results have been promising.
National Vaccine Trials
While DHVI researchers are working on new-and-improved vaccine designs, they are also participating in the nationwide effort to get the first wave of vaccine candidates evaluated as quickly as possible by serving as a clinical trial site. Phase 2 and 3 clinical trials require tens of thousands of volunteers at multiple sites across the country. DHVI enrolled more than 80 volunteers for the Phase 2/3 trial of the Pfizer vaccine candidate, and is now enrolling even more participants for a trial of AstraZeneca’s vaccine.
Walter, who is leading this effort as head of the DHVI Clinical Trials Unit, says, “DHVI is pretty well positioned because of its experience with HIV, ranging from vaccine discovery to the ability to implement clinical trials. Shifting to COVID was challenging, but we had the resources to do it.”
Walter also leads Duke’s participation in the nationwide series of trials to test treatments for patients hospitalized with COVID-19. The first trial studied remdesivir alone, and subsequent trials tested it in combination with other medicines. “The first study showed decreased time hospitalized for patients who got remdesivir, hence it became standard of care,” Walter says.
Testing and Diagnostics
Beyond vaccines, DHVI is also pursuing other avenues, including testing and diagnostics. Thomas Denny, DHVI chief operating officer, and his lab assisted with testing in the early days of the pandemic, when clinical labs were overwhelmed. Denny led a team at DHVI that also designed and implemented the surveillance testing of students, staff, and faculty being used on campus this fall. As part of the campus testing program, DVHI has processed almost 100,000 tests since August 2.

Denny is also working on designing more sensitive assays that can determine not just whether the virus is present or not, but in what amounts. “With a lot of viral infections, like HIV, we’ve learned over the years that being able to quantify the viral amount has been useful as a signal with respect to disease prognosis or response to therapy,” he says. If the same is true for COVID-19, that information could be used to guide clinical decisions. Denny is analyzing samples from COVID-infected adults and children who are participating in observational studies at Duke. He will compare the results of his assays with notes on their clinical condition to look for correlations between viral load and disease progression.
Denny’s lab also developed assays to look for antibodies to SARS-CoV-2 in the blood, which could, among other things, be used in seroprevalence studies to show how many people have recovered from COVID-19.
The Immune Response as Diagnosis
Christopher Woods, MD, MPH, professor of medicine, and his team are coming at diagnostics from a different direction—looking at the immune response. The idea is that samples from an infected person will contain not only the pathogen, but also biochemical signals of the immune response. In fact, the immune signals may be easier to detect in early stages of infection than the pathogen, which is only just beginning to multiply. Woods has a track record in this area: he and his team have been able to distinguish viral from bacterial infections based on the immune response, and to identify infections 36 to 48 hours before the onset of symptoms.
“We have not had great success [in the past] being able to distinguish different types of viral respiratory infections,” he says. “Until COVID.” He and his team have found a unique signature in blood samples that indicates the immune system is mounting a response to SARS-CoV-2 infection. The samples used in that study were from people who were past the early stages of infection, but Woods is planning future studies to see if the signature is present in the pre-symptomatic phase of COVID-19. If so, a diagnostic test for that signal could help curb the spread of the disease and allow earlier treatment.
The Immune Response to COVID-19 in Children
Sallie Permar, MD, PhD, professor of pediatrics, molecular genetics & microbiology, immunology, and pathology, is working to understand the immune response to COVID-19 in children. Although children do get the disease, they are more likely to have no or few symptoms than adults. However, some children experience a severe inflammatory reaction to COVID-19, called Multisystem Inflammatory Syndrome in Children (MIS-C).
“Not only do we want to understand what about infant or pediatric infections leads to the lack of disease during the acute infection,” Permar says, “but also what are the factors that lead to post-infection inflammatory syndrome?”
To help answer some of these questions, Permar and Maria Blasi, PhD, assistant professor of medicine, are doing a study in non-human primates to track the immune response over the course of infection in adults and infants. They are also studying adult and infant lung cells in the lab to see how the cells respond to infection.
DHVI researchers are also studying children in several ongoing observational trials. These trials include infected children as well as children who are uninfected (at least initially) but living with someone who is. The children are being followed over time to learn more about immune activity and clinical symptoms during infection, recovery, and beyond. “We don’t yet know what a long-term response to the coronavirus is,” Permar says. “We’ll be studying them for at least a year.”
A Vaccine for Future Coronavirus Pandemics?
While the Haynes, Saunders, and Sempowski labs were isolating antibodies from COVID-infected individuals, they also looked at a sample from a person who had been infected with another pandemic-causing coronavirus—Severe Acute Respiratory Syndrome (SARS)—in 2003. They discovered that some of the SARS antibodies from that individual also neutralized SARS-CoV-2.
That raised a tantalizing question: Might it be possible to design a vaccine that elicits cross-protective antibodies? Such a vaccine—a pan-coronavirus vaccine—would protect against multiple coronaviruses, including Middle East Respiratory Syndrome (MERS), which emerged in 2012, as well as other as-yet-unknown coronaviruses.
“SARS-CoV-2 is not a one-off event,” Saunders says. “There seems to be a coronavirus pandemic every eight to ten years. We’re looking at the future pandemics and trying to predict what that will look like and to see if we can generate immunity for those types of viruses.”
DHVI has already begun working with scientists at UNC-Chapel Hill on a pan-coronavirus vaccine.
“It’s only a matter of time before the next coronavirus outbreak,” Haynes says, “and we will be ready for it.”
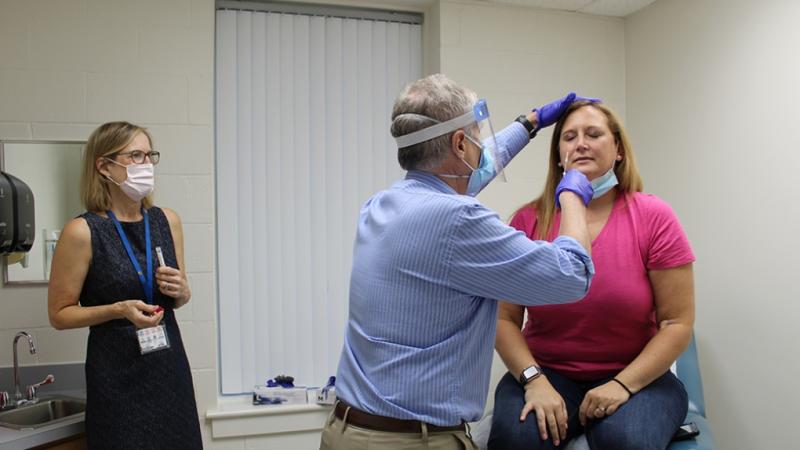
Top photo: Emmanuel "Chip" Walter, MD, head of the DHVI Clincal Trials Unit, gives a nasal swab to Kristin Weaver, a healthy participant in the Pfizer vaccine clinical trial. DHVI enrolled more than 80 volunteers for the Phase 2/3 trial of the Pfizer vaccine candidate. Photo by Lindsay Key.
Mary-Russell Roberson is a freelance writer in Durham. She covers the geriatrics and aging beat for the Department of Medicine in the Duke University School of Medicine.
