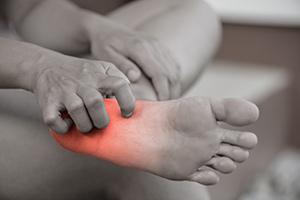
Chemotherapy is a proven strategy for killing cancer cells, but treatment can affect the small sensory nerves in the feet and hands, causing tingling, pain, numbness, and hypersensitivity. Some patients experience sensitivity so extreme that they have difficulty wearing socks, shoes, long pants and long sleeves.
In the clinic, when chemotherapy patients tell Duke neurologist Wolfgang Liedtke, MD, PhD, about pain in their hands and feet, he prescribes an individualized assortment of anti-pain medicine for treatment which typically includes anti-seizure or mood stabilizing tablets, plus topical treatments (such as gels, ointments) containing numbing and anti-oxidant agents. However, topical gels are often not strong enough, and tablets readily cause systemic side effects, adding to the “brain fog” of chemotherapy.
Liedtke has treated patients suffering from chronic “therapy-refractory” pain for many years now. By working closely with his patients, he understood the opportunity that the unmet medical need that chemotherapy-induced painful polyneuropathy (CIPN) represents.
“When it comes to chemotherapy-associated pain, we want to alleviate pain for the obvious reasons,” Liedtke said. “But also because reduced pain allows a patient to fully implement their treatment, increasing the likelihood of survival.”
The pain and discomfort of CIPN that chemotherapy patients experience likely occurs because sensory nerve cells have very long extensions into hands and feet that can be injured by an excess of calcium ions.
In 2000, Liedtke discovered a gatekeeper that allows calcium into sensory nerve cells—a signaling molecule known as TRPV4. In his lab at Duke, Liedtke began experimenting with different sets of compounds to see if they could inhibit TRPV4 so that less calcium enters the nerve cells.
He discovered a set of compounds that so far have been effective in reducing calcium influx into sensory nerve cells in relevant preclinical animal models and also in isolated cells including human cells.
 In 2017, Liedtke and partner George “Barney” Koszalka, PhD, founded a company called TRPblue with the mission of developing these compounds into a topical treatment, or skin cream, to prevent CIPN.
In 2017, Liedtke and partner George “Barney” Koszalka, PhD, founded a company called TRPblue with the mission of developing these compounds into a topical treatment, or skin cream, to prevent CIPN.
The small business spin off was supported by Duke’s Office of Licensing and Ventures (OLV) and by Durham-based Medblue Incubator. Liedtke and Koszalka have partnered with Carlos Dedesma, PhD, MBA, a Mentor-in-Residence for OLV, to help ensure the company’s success.
In order to advance to human clinical trials, the new TRPblue compound will have to undergo extensive testing of its chemical, pharmaco-kinetic, and pharmaco-toxicologic properties before being green-lighted for first testing in humans, said Liedtke.
“What Wolfgang has developed is an interesting set of compounds which we hope to advance fairly rapidly into the clinic,” said Koszalka. “It’s not systemic, it’s a topical agent, and that has some very particular advantages in its development cycle as far as shortening the development cycle and getting it into Phase I, Phase II clinical trials fairly rapidly.”
TRPBlue is a featured innovator at the second annual Invented at Duke Celebration, held from 4 to 7 p.m. on Thursday, November 8, 2018 at Penn Pavilion on Duke’s campus. The event is sponsored by Duke’s Office of Licensing and Ventures, and the Duke Innovation and Entrepreneurship Initiative.