People are great at detecting cold temperatures and also the cool sensation induced by natural substances like menthol, which is common in remedies used to soothe aching muscles. But it hasn’t been entirely clear how we do this.
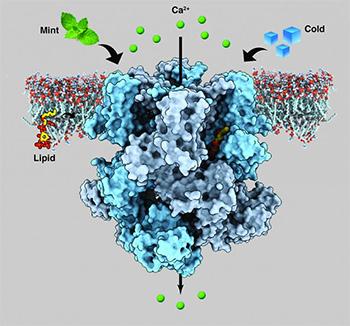
About a year ago, a group of researchers led by Seok-Yong Lee, Associate Professor of Biochemistry in the Duke University School of Medicine, figured out the architecture of the human and animal cold-sensing protein, an ion channel called TRPM8, which gave them some insight into its function but also raised more questions.
Now, Lee's team has determined the structure TRPM8 assumes when it is bound to menthol and to another synthetic cooling agent called icilin. The findings, which will appear in Science on Feb. 8, could pave the way toward new treatments for chronic pain and migraine and help patients who suffer from extreme cold sensitivity.
In an attempt to better understand TRPM8, Lee and colleagues used a technique called cryo-electron microscopy (cryo_EM), which is new to Duke.
The strategy involves purifying the protein from cells, flash freezing it, and then bouncing electrons off of the sample embedded in ice in a giant machine called a Titan Krios. The cryo-EM made a composite of images of more than a million protein particles in various orientations and then computed a high-resolution three-dimensional structure for TRPM8.
Read entire article at Duke Today